Aging Population
The aging population in the US is a critical driver for the medical devices market. As individuals age, they often experience a higher incidence of chronic diseases and conditions that require medical intervention. This demographic shift is projected to increase the demand for various medical devices, including diagnostic equipment, surgical instruments, and home healthcare devices. By 2030, it is estimated that around 20% of the US population will be over 65 years old, which could lead to a substantial rise in the medical devices market. The need for devices that cater to the elderly, such as mobility aids and monitoring systems, is likely to expand, thereby influencing market growth significantly.
Regulatory Support
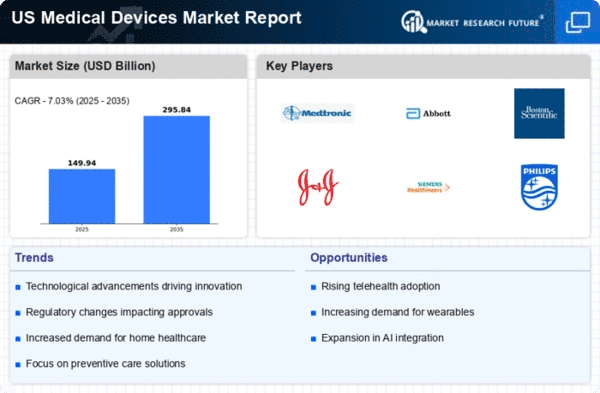
Regulatory support is a significant driver for the medical devices market, as it ensures the safety and efficacy of medical products. The US Food and Drug Administration (FDA) plays a crucial role in this regard, providing a framework for the approval and monitoring of medical devices. Recent initiatives aimed at expediting the approval process for innovative devices have the potential to enhance market growth. For instance, the FDA's Breakthrough Devices Program allows for faster access to devices that address unmet medical needs. This regulatory environment fosters innovation and encourages investment in the medical devices market, which is expected to grow at a CAGR of around 5% over the next several years.
Technological Advancements
Technological advancements play a pivotal role in shaping the medical devices market. Innovations such as minimally invasive surgical techniques, advanced imaging technologies, and smart wearable devices are transforming patient care. The integration of artificial intelligence and machine learning into medical devices enhances diagnostic accuracy and treatment efficacy. In 2025, the market for imaging devices alone is projected to reach approximately $40 billion, reflecting the growing reliance on technology in healthcare. These advancements not only improve patient outcomes but also streamline operations within healthcare facilities, thereby driving the demand for new and upgraded medical devices.
Rising Healthcare Expenditure
Rising healthcare expenditure in the US is a driving force behind the medical devices market. Increased spending on healthcare services and technologies reflects a growing commitment to improving patient care and outcomes. In 2025, total healthcare spending in the US is projected to exceed $4 trillion, with a significant portion allocated to medical devices. This trend indicates a robust market environment where healthcare providers are more willing to invest in advanced medical technologies. The expansion of insurance coverage and reimbursement policies further supports this growth, as it allows for greater access to innovative medical devices for patients.
Increased Focus on Preventive Care
An increased focus on preventive care is emerging as a vital driver for the medical devices market. Healthcare providers are increasingly prioritizing preventive measures to reduce the incidence of chronic diseases and improve overall population health. This shift is leading to a higher demand for diagnostic and monitoring devices that facilitate early detection and intervention. For instance, wearable health technology, such as fitness trackers and remote monitoring devices, is gaining popularity among consumers. The medical devices market is likely to benefit from this trend, as more individuals seek tools that empower them to take charge of their health and well-being.


















Leave a Comment