The US IV Fluid Monitoring Devices Market is characterized by a dynamic competitive landscape wherein various firms compete to provide innovative solutions that enhance patient safety and improve clinical outcomes in healthcare settings. As the healthcare industry increasingly emphasizes patient-centric care and efficiency, the demand for advanced fluid monitoring devices continues to grow. This market includes a range of products such as infusion pumps and monitoring systems that ensure accurate drug delivery while reducing the likelihood of errors.
Companies in this sector must navigate regulatory challenges, integrate technological advancements, and respond to evolving customer needs to maintain their competitive edge. Collaborative efforts, strategic partnerships, and investments in research and development are crucial for companies aiming to establish a strong presence in this thriving market.
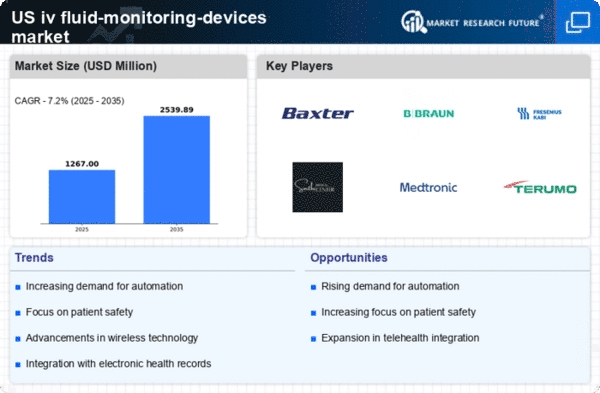
Key Players in the US IV Fluid Monitoring Devices Market
C.R. Bard is a notable player in the US IV Fluid Monitoring Devices Market, recognized for its innovation and high-quality products. The company's strengths lie in its comprehensive portfolio of IV access devices, along with a commitment to enhancing patient care through reliable and efficient products. With a strong focus on technology, C.R. Bard continually improves its offerings, which include advanced catheter systems and integrated monitoring solutions.
The company's established reputation and robust distribution network enable it to maintain a significant market presence, providing healthcare providers with trusted solutions that enhance overall treatment effectiveness. Through ongoing innovation and strategic investments, C.R. Bard is well positioned to meet the growing demands of the US healthcare market, solidifying its leading role in the IV fluid monitoring space.
Smiths Medical, another key player in the US IV Fluid Monitoring Devices Market, offers a diverse array of products and services aimed at improving patient outcomes in critical care environments. The company's portfolio includes infusion pumps, vascular access devices, and safety products designed to minimize risks associated with intravenous therapy. Smiths Medical’s strengths lie in its emphasis on both product quality and technological advancement, which fosters trust among healthcare practitioners.
The company has engaged in strategic mergers and acquisitions that have bolstered its market position and expanded its product lines, thereby enhancing its competitiveness in the US market. Smiths Medical’s ongoing commitment to innovation, customer service, and partnership-building further strengthens its footprint in the IV fluid monitoring sector, allowing it to effectively address the needs of healthcare facilities and ultimately improve patient care in the US.