Technological Advancements
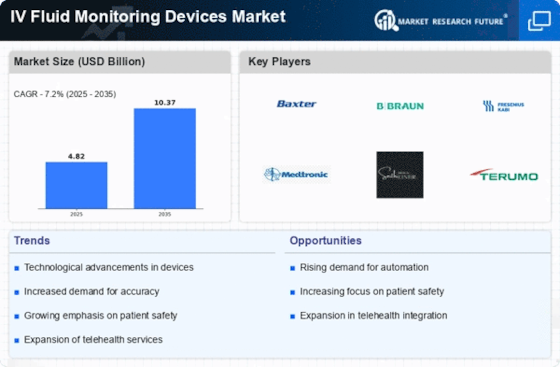
The IV Fluid Monitoring Devices Market is experiencing a surge in technological advancements that enhance the efficiency and accuracy of fluid management. Innovations such as smart infusion pumps and integrated monitoring systems are becoming increasingly prevalent. These devices utilize advanced algorithms and connectivity features to provide real-time data, which is crucial for patient care. The market is projected to grow at a compound annual growth rate (CAGR) of approximately 8% over the next few years, driven by the demand for improved patient outcomes and reduced medication errors. Furthermore, the integration of artificial intelligence and machine learning into these devices is likely to revolutionize the way healthcare providers monitor and manage IV fluids, thereby increasing the overall effectiveness of treatment protocols.
Regulatory Support and Standards
The IV Fluid Monitoring Devices Market is bolstered by regulatory support and the establishment of stringent standards aimed at ensuring device safety and efficacy. Regulatory agencies are actively working to create frameworks that facilitate the approval and monitoring of IV fluid devices, which in turn fosters innovation and market growth. Compliance with these regulations not only enhances the credibility of manufacturers but also instills confidence among healthcare providers and patients. As the industry adapts to these evolving standards, it is likely to witness an influx of new products designed to meet regulatory requirements. This supportive environment is expected to drive the expansion of the IV fluid monitoring devices market, as stakeholders recognize the importance of adhering to established guidelines for improved patient care.
Increased Focus on Patient Safety
The IV Fluid Monitoring Devices Market is witnessing an increased focus on patient safety, which is driving the adoption of advanced monitoring technologies. Healthcare institutions are prioritizing the implementation of systems that minimize the risk of medication errors and adverse events associated with IV fluid administration. Regulatory bodies are also emphasizing the importance of safety standards, leading to the development of more sophisticated monitoring devices. As a result, hospitals and clinics are investing in IV fluid monitoring solutions that provide alerts for potential issues, thereby enhancing patient safety. This trend is expected to contribute to the growth of the market, as healthcare providers recognize the value of investing in technologies that protect patients and improve clinical outcomes.
Growing Demand for Home Healthcare
The IV Fluid Monitoring Devices Market is experiencing a shift towards home healthcare, driven by the growing demand for patient-centered care. As more patients prefer receiving treatment in the comfort of their homes, the need for portable and user-friendly IV fluid monitoring devices is increasing. This trend is supported by advancements in technology that allow for remote monitoring and data transmission, enabling healthcare providers to oversee patient care from a distance. The home healthcare market is projected to expand significantly, with estimates suggesting a growth rate of around 10% annually. Consequently, the IV fluid monitoring devices market is likely to benefit from this trend, as manufacturers develop solutions tailored for home use, ensuring that patients receive the necessary care without frequent hospital visits.
Rising Incidence of Chronic Diseases
The IV Fluid Monitoring Devices Market is significantly influenced by the rising incidence of chronic diseases, which necessitate continuous and precise fluid management. Conditions such as diabetes, heart disease, and renal failure often require patients to receive IV fluids regularly. As the prevalence of these diseases continues to rise, the demand for effective monitoring solutions is expected to increase correspondingly. According to recent statistics, chronic diseases account for nearly 70% of all deaths worldwide, underscoring the urgent need for reliable IV fluid management systems. This trend is likely to propel the IV fluid monitoring devices market, as healthcare providers seek to enhance patient care and ensure optimal fluid administration.