Global Travel and Trade Dynamics
The dynamics of The chikungunya vaccine market significantly. As international travel increases, the risk of chikungunya transmission also rises, particularly in regions where the virus is endemic. Travelers returning to the US from affected areas may introduce the virus, leading to localized outbreaks. This potential for outbreaks may prompt health authorities to recommend vaccination for travelers, thereby increasing demand for chikungunya vaccines. Additionally, the interconnectedness of global trade may facilitate the distribution of vaccines, making them more accessible to populations at risk. Thus, the chikungunya vaccine market could see growth driven by the need for preventive measures in the context of global mobility.
Advancements in Vaccine Technology
Technological advancements in vaccine development are poised to enhance the chikungunya vaccine market. Innovations such as mRNA technology and viral vector platforms have shown promise in creating effective vaccines against various infectious diseases. These advancements may lead to the development of more effective and safer chikungunya vaccines, which could significantly boost public confidence in vaccination. Furthermore, the ability to produce vaccines more rapidly and efficiently could address the urgent need for immunization in response to outbreaks. As a result, the chikungunya vaccine market may see an influx of new products that leverage these technologies, potentially increasing market share and accessibility.
Rising Incidence of Chikungunya Cases
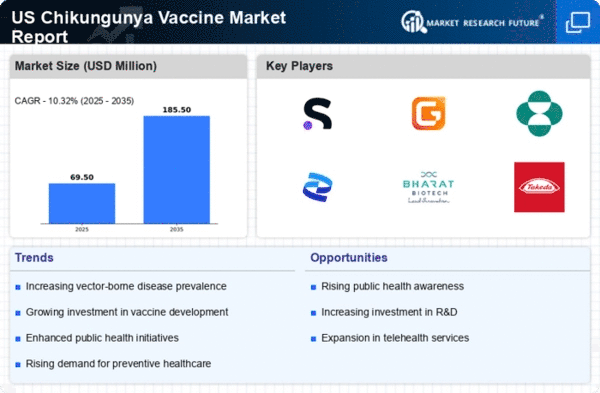
The chikungunya vaccine market is likely to experience growth due to the increasing incidence of chikungunya cases in the US. Reports indicate that the number of chikungunya infections has risen significantly, with thousands of cases reported annually. This surge in cases raises public awareness and concern, prompting health authorities to prioritize vaccine development and distribution. The Centers for Disease Control and Prevention (CDC) has noted that the disease can lead to severe joint pain and other debilitating symptoms, which further emphasizes the need for effective vaccination strategies. As the population becomes more aware of the risks associated with chikungunya, the demand for vaccines is expected to increase, thereby driving the chikungunya vaccine market forward.
Increased Public Awareness and Education
Public awareness campaigns regarding chikungunya and its potential health impacts are likely to drive the chikungunya vaccine market. As educational initiatives increase, more individuals are becoming informed about the disease, its transmission, and the benefits of vaccination. Health organizations are actively promoting the importance of vaccines in preventing chikungunya, which may lead to higher vaccination rates. This heightened awareness could translate into increased demand for vaccines, as individuals seek protection against the disease. Consequently, the chikungunya vaccine market may benefit from a more informed public that recognizes the value of immunization in safeguarding health.
Government Support and Policy Initiatives
Government support plays a crucial role in shaping the chikungunya vaccine market. Federal and state health agencies are increasingly recognizing the importance of vaccination in controlling vector-borne diseases. Initiatives aimed at funding research and development of chikungunya vaccines are being implemented, which may lead to increased investment in this area. For instance, the National Institutes of Health (NIH) has allocated substantial resources to support vaccine trials and studies. Such government backing not only facilitates the development of effective vaccines but also encourages private sector involvement, thereby expanding the chikungunya vaccine market. The collaboration between public and private entities is likely to enhance the overall efficacy and reach of vaccination programs.