Rising Demand for Customization
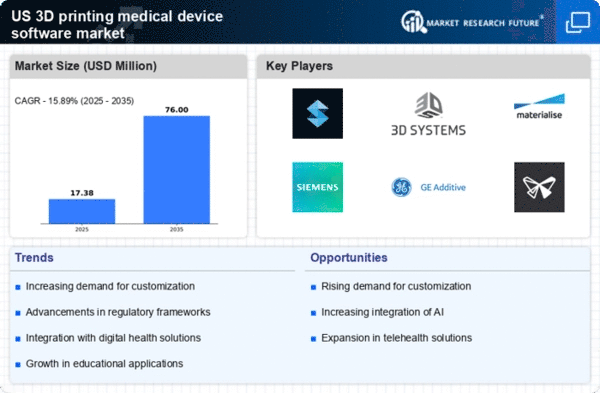
Customization is becoming a pivotal driver in the 3d printing-medical-device-software market. Healthcare providers are increasingly seeking tailored solutions to meet the specific needs of patients, which traditional manufacturing methods often cannot accommodate. The ability to produce patient-specific implants and prosthetics through 3D printing is revolutionizing treatment approaches. Reports indicate that the demand for customized medical devices is expected to grow at a CAGR of over 25% in the coming years. This trend is fueled by the desire for improved patient outcomes and satisfaction, as personalized devices can lead to better fit and functionality. Consequently, the 3d printing-medical-device-software market is positioned to benefit significantly from this shift towards customization in healthcare.
Regulatory Support and Frameworks
Regulatory support plays a crucial role in shaping the 3d printing-medical-device-software market. As the technology matures, regulatory bodies are developing frameworks to ensure the safety and efficacy of 3D printed medical devices. The FDA has been actively working on guidelines that facilitate the approval process for innovative medical technologies, which is essential for fostering industry growth. This regulatory clarity encourages manufacturers to invest in 3D printing technologies, knowing that there is a pathway for compliance. The establishment of clear regulations is expected to enhance market confidence and stimulate innovation, thereby propelling the 3d printing-medical-device-software market forward.
Cost Efficiency and Waste Reduction
Cost efficiency is a critical factor driving the growth of the 3d printing-medical-device-software market. Traditional manufacturing processes often involve high material waste and extended lead times, whereas 3D printing allows for on-demand production, significantly reducing waste. This efficiency not only lowers production costs but also enables healthcare facilities to manage their budgets more effectively. A study suggests that 3D printing can reduce costs by up to 50% in certain applications, making it an attractive option for hospitals and clinics. As healthcare systems continue to seek ways to optimize expenditures while maintaining quality, the 3d printing-medical-device-software market is likely to see increased investment and adoption.
Technological Advancements in 3D Printing
The 3D printing medical device software market is experiencing rapid technological advancements that enhance the capabilities of 3D printing. Innovations in materials, such as biocompatible polymers and metals, are expanding the range of applications for medical devices. For instance, the introduction of advanced software algorithms allows for more precise modeling and simulation, which can lead to better patient outcomes. According to recent data, the market for 3D printing in healthcare is projected to reach approximately $3.5 billion by 2026, indicating a robust growth trajectory. These advancements not only improve the quality of medical devices but also reduce production costs, making them more accessible to healthcare providers. As technology continues to evolve, the 3d printing-medical-device-software market is likely to see increased adoption across various medical fields.
Increased Investment in Healthcare Innovation
Investment in healthcare innovation is a significant driver for the 3d printing-medical-device-software market. Venture capital and private equity firms are increasingly recognizing the potential of 3D printing technologies in transforming medical device manufacturing. In recent years, funding for startups focused on 3D printing solutions has surged, with investments reaching over $1 billion in 2025 alone. This influx of capital is enabling companies to accelerate research and development efforts, leading to the introduction of novel medical devices and software solutions. As the healthcare landscape evolves, the 3d printing-medical-device-software market is likely to benefit from sustained investment, fostering a culture of innovation and growth.