Transplant Diagnostics Market Summary
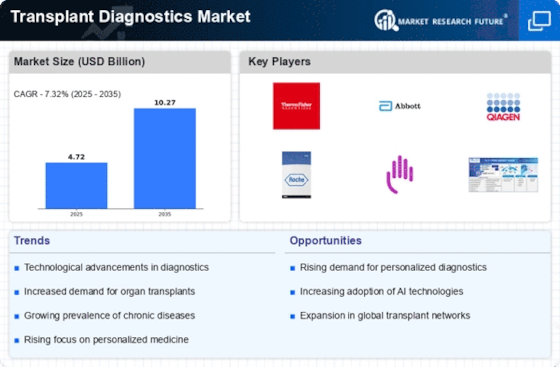
As per Market Research Future analysis, the Transplant Diagnostics Market Size was estimated at 8.4 USD Billion in 2025. The Transplant Diagnostics industry is projected to grow from 9.1 USD Billion in 2026 to 18.0 USD Billion by 2035, exhibiting a compound annual growth rate (CAGR) of 7.9% during the forecast period 2026 - 2035
Key Market Trends & Highlights
The Transplant Diagnostics Market is poised for substantial growth driven by technological advancements and increasing demand for personalized medicine.
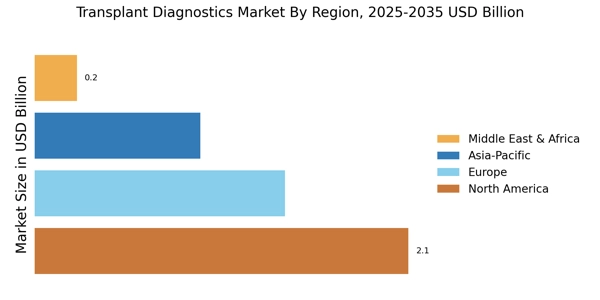
- Technological advancements are enhancing the accuracy and efficiency of transplant diagnostics, particularly in North America.
- The Asia-Pacific region is emerging as the fastest-growing market, driven by increasing healthcare investments and rising awareness.
- Instruments represent the largest segment of the market, while reagents and consumables are experiencing the fastest growth due to their essential role in diagnostics.
- The rising incidence of organ transplants and advancements in diagnostic technologies are key drivers propelling market expansion.
Market Size & Forecast
| 2025 Market Size | 8.4 (USD Billion) |
| 2035 Market Size | 18.0 (USD Billion) |
| CAGR (2026 - 2035) | 7.9% |
Major Players
Thermo Fisher Scientific (US), Abbott Laboratories (US), Qiagen (DE), Hoffmann-La Roche (CH), Bristol-Myers Squibb (US), Immucor company, HLA Typing (US), GenDx (NL), Natera (US), CareDx (US)