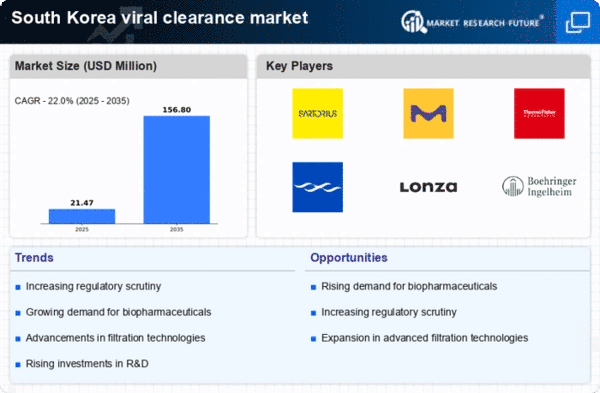
The South Korea Viral Clearance Market has witnessed significant growth and development in recent years, driven by increasing demand for effective viral removal and inactivation technologies in biopharmaceutical manufacturing. This sector has become crucial as industries recognize the importance of ensuring the safety and efficacy of biological products. Companies operating within this market are continuously innovating and expanding their service offerings to meet stringent regulatory requirements and consumer expectations. The competitive landscape is characterized by a surge in research and development efforts, collaborations, and partnerships aimed at enhancing viral clearance solutions.
As entities strive to position themselves as leaders in this domain, market participants are focusing on technology advancements, quality assurance, and comprehensive service portfolios that cater to a variety of applications, including vaccines, monoclonal antibodies, and cell and gene therapies.SK Bioscience stands out in the South Korea Viral Clearance Market due to its robust research and development capabilities and strong emphasis on innovation. The company has established itself as a significant player by leveraging its cutting-edge technologies to develop viral clearance products that meet high international standards.
With a commitment to quality and safety, SK Bioscience has successfully implemented a range of viral inactivation processes tailored for the biopharmaceutical industry. The company’s strategic investments in state-of-the-art manufacturing facilities have further enhanced its market presence, enabling it to respond effectively to the growing demand for viral clearance solutions.
Additionally, SK Bioscience’s partnerships with various academic and research institutions bolster its position in the market, facilitating access to the latest findings and technologies that enhance its product offerings.Celltrion is another key player in the South Korea Viral Clearance Market, known for its comprehensive approach to biopharmaceutical development that includes a strong focus on viral clearance processes. The company offers a variety of key products and services that align with industry standards for safety and efficacy, particularly in the development of monoclonal antibodies and biosimilars.
Celltrion's market presence is strengthened by its innovative manufacturing capabilities and a commitment to maintaining regulatory compliance. The company has successfully executed mergers and acquisitions that have expanded its technological base and service capabilities, allowing it to cover an extensive range of viral clearance needs in the region. Through its strategic initiatives, Celltrion continues to enhance its competitive edge in the market, thereby contributing significantly to the advancement of safe and effective biopharmaceutical solutions in South Korea.