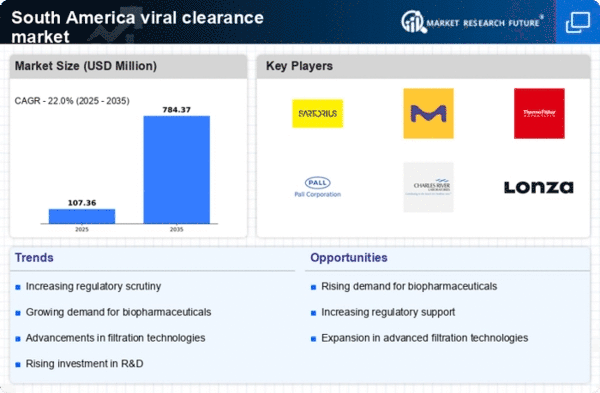
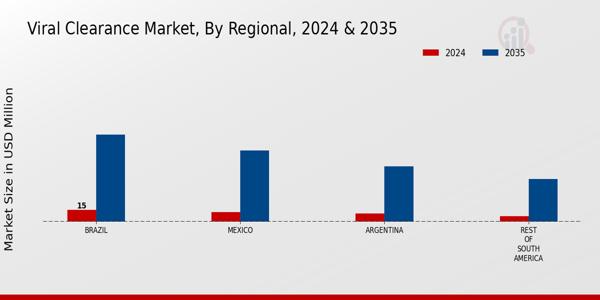
The South America Viral Clearance Market has witnessed significant growth and evolution in recent years, driven by increasing concerns over viral contamination in biopharmaceutical manufacturing and the necessity for stringent regulatory compliance. The market is characterized by a diverse range of players, each striving to gain a competitive edge through innovative technologies, expanding product offerings, and strategic collaborations. As the region continues to strengthen its healthcare infrastructure and biotechnology capabilities, companies are increasingly focusing on enhancing their viral clearance processes to meet the demands of safety and efficacy in therapeutic products.
The competitive landscape is dynamic, with organizations leveraging their expertise in filtration, chromatography, and inactivation methodologies to carve out a substantial market presence.Sartorius has established itself as a formidable player in the South America Viral Clearance Market by leveraging its advanced technologies and comprehensive service offerings. The company is recognized for its robust portfolio of products, which includes innovative filtration solutions and dedicated support for biopharmaceutical manufacturers. Sartorius has been adept at forming strategic partnerships and collaborations to bolster its market reach and enhance its product capabilities.
With a focus on quality and regulatory compliance, Sartorius has built a strong reputation for reliability and efficiency in viral clearance processes, positioning itself as a preferred choice among bioprocessing clients in the region. The company's technical expertise and commitment to innovation enable it to cater effectively to the evolving needs of the South American biopharmaceutical sector.Pall Corporation also holds a significant place in the South America Viral Clearance Market, offering a wide range of solutions that address the challenges associated with viral contamination.
The company's product lineup includes cutting-edge filtration systems, viral inactivation technologies, and comprehensive services that cater to the requirements of bioprocessing facilities. Pall Corporation's strength lies in its extensive experience and technological prowess, which have allowed it to establish a solid market presence across various biopharmaceutical sectors in South America. The company has pursued several strategic mergers and acquisitions to bolster its capabilities and expand its offerings in viral clearance technologies.
By aligning itself with local and regional firms, Pall Corporation has enhanced its ability to deliver tailored solutions that meet the specific needs of its South American customers, reinforcing its competitive position in the market.