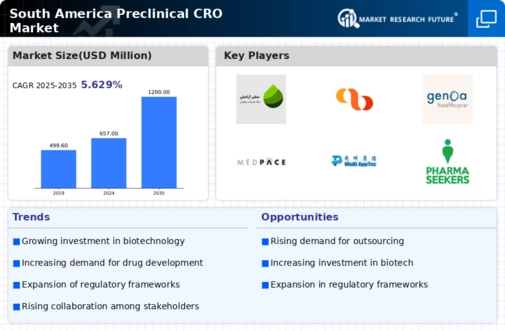
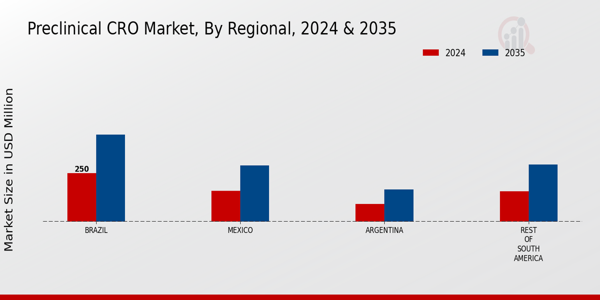
The South America Preclinical CRO Market has become increasingly competitive, fueled by an ever-expanding demand for pharmaceutical research and development services. The evolving landscape of biotechnology and rising investment in drug development have highlighted the essential role of Contract Research Organizations (CROs) in preclinical research processes.
The market is characterized by a mix of established players and emerging firms, each striving to offer innovative solutions that cater to the unique regulatory and research challenges of the region. As companies focus on building comprehensive service portfolios, they aim to leverage local partnerships and enhance operational efficiencies, thereby creating a dynamic environment that significantly shapes competition and collaboration among stakeholders within South America.
Safire has established a strong presence in the South America Preclinical CRO Market, known for its expertise in providing tailored solutions that cater specifically to the needs of various clients in the biopharmaceutical industry. One of the major strengths of Safire includes its deep understanding of the local regulatory landscape, which allows the company to navigate compliance issues effectively while accelerating timelines for drug development.
In addition, Safire has garnered a reputation for its robust scientific capabilities, allowing for a diverse range of preclinical studies across multiple therapeutic areas. The firm's ability to form strategic alliances and collaborations with local academic institutions and industry partners further solidifies its competitive advantage, positioning it as a trusted partner in preclinical research across South America.
Syneos Health, with its broad portfolio of services in the South America Preclinical CRO Market, has carved out a notable space for itself by offering comprehensive solutions that encompass both therapeutic and operational expertise. The company is particularly strong in integrating scientific and commercial insights to expedite the drug development process.
One of Syneos Health's key strengths lies in its ability to provide end-to-end services, which enhances clients’ capabilities in conducting preclinical studies. The firm has also made strategic investments through mergers and acquisitions to bolster its presence and operational capabilities in the region. These moves have allowed Syneos Health to expand its service offerings and enhance its market position.
With key products that support a wide range of preclinical programs, the company's innovative approach positions it well to meet the growing demands of the biopharmaceutical sector in South America.





















Leave a Comment