Investment in Biotechnology
France's preclinical cro market is significantly influenced by the growing investment in biotechnology. The French government has been actively promoting biotechnology initiatives, leading to an influx of funding for research and development. In 2025, the biotechnology sector is expected to receive over €1 billion in investments, which will likely enhance the capabilities of preclinical CROs. This financial support enables these organizations to adopt cutting-edge technologies and methodologies, thereby improving the quality and efficiency of preclinical studies. Consequently, the preclinical cro market stands to gain from this investment, as it fosters innovation and accelerates the development of novel therapeutics.
Focus on Personalized Medicine
The shift towards personalized medicine is reshaping the landscape of the preclinical cro market in France. As healthcare providers increasingly recognize the importance of tailored treatments, there is a growing demand for preclinical studies that support the development of personalized therapies. This trend is expected to drive the preclinical cro market, as CROs adapt their services to meet the specific needs of clients focused on individualized treatment approaches. By 2025, it is anticipated that personalized medicine will account for approximately 30% of the total drug development pipeline, underscoring the critical role of preclinical CROs in this evolving paradigm.
Regulatory Changes and Compliance
The preclinical cro market in France is also shaped by evolving regulatory frameworks and compliance requirements. Regulatory agencies are increasingly emphasizing the need for rigorous preclinical testing to ensure the safety and efficacy of new drugs. This shift necessitates that preclinical CROs enhance their operational standards and methodologies to align with these regulations. In 2025, it is projected that compliance-related expenditures in the preclinical cro market will increase by 15%, reflecting the heightened focus on regulatory adherence. As a result, CROs that can effectively navigate these changes are likely to gain a competitive advantage in the market.
Rising Demand for Drug Development
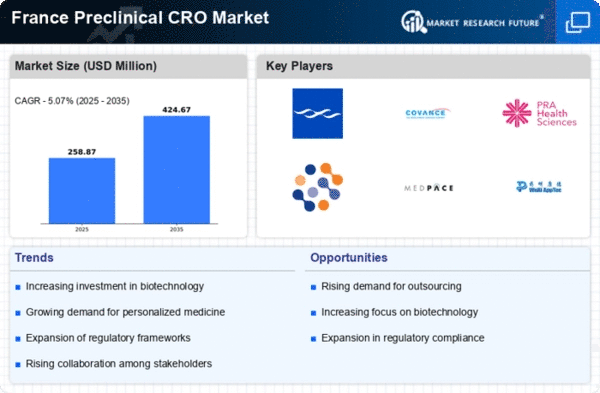
The preclinical cro market in France is experiencing a notable surge in demand for drug development services. This trend is largely driven by the increasing need for innovative therapies to address various health challenges. As pharmaceutical companies seek to expedite their research and development processes, the reliance on preclinical contract research organizations (CROs) has intensified. In 2025, the market is projected to grow at a CAGR of approximately 8%, reflecting the industry's response to the urgent need for efficient drug discovery and development. The preclinical cro market is thus positioned to play a crucial role in facilitating the advancement of new therapeutics, ultimately benefiting patients and healthcare systems alike.
Emergence of Innovative Technologies
The integration of innovative technologies is a key driver of growth in the preclinical cro market in France. Advancements in areas such as artificial intelligence, machine learning, and high-throughput screening are revolutionizing the way preclinical studies are conducted. These technologies enable CROs to streamline processes, reduce costs, and enhance the accuracy of results. By 2025, it is estimated that the adoption of such technologies will lead to a 20% reduction in the time required for preclinical studies. Consequently, the preclinical cro market is poised to benefit from these innovations, as they facilitate faster and more efficient drug development.