Market Trends
Key Emerging Trends in the Serotonin Syndrome Market
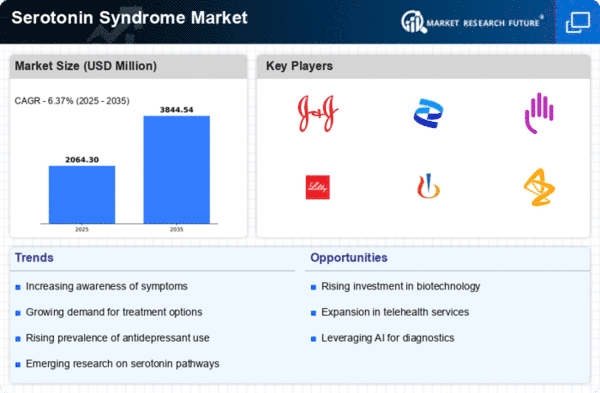
Serotonin syndrome market trends reflect the changing face of healthcare and pharmaceuticals as our understanding and awareness about this condition continues to develop. It is a potentially deadly condition that results from an excess amount of serotonin in the body. There has been notable changes in the market for therapies and interventions related to serotonin syndrome recently, brought by medical research advancements, diagnostic technology improvements, as well as therapeutic approaches evolution.
Rising prevalence is another key trend within the serotonin syndrome market. As people become more enlightened about serotonin syndrome, health providers are putting more emphasis on early detection and diagnosis. This has in turn led to an increase in demand for diagnostic tools and tests that can detect quickly if there is serotonergic crisis. Consequently, the market responded with developing sophisticated diagnostic technologies accessible by all facilitating quicker syndromes identification.
The other important trend is increased number of treatment options available to patients suffering from serotonin syndrome. So far treatment modalities have largely focused on cessation of serotonergic drugs use with supportive care being offered afterwards. Nonetheless, the last couple of years have seen new therapeutics aimed at addressing underlying neurochemical imbalances associated with elevated level of serotonin released into blood emerge. To this effect different pharmaceutical firms are currently involved in various research initiatives geared at discovering newer drugs that could be used effectively but safely treat patients undergoing serotonin syndrome.
The market for serotonin syndrome also reflects a growing emphasis on patient education and awareness. Health practitioners are increasingly engaged in educating both their colleagues as well as patients in relation to risk factors, symptoms and preventive measures related to SSRI toxicity. This corresponds with development of educational materials including online resources besides public sensitization campaigns which means that we now have a much more aware community within this field.
Globalization has greatly influenced trends regarding cases of Serotonin Syndrome through drug markets worldwide since it encourages manufacturers to cater for diverse populations as they look outwards towards entire world markets where people may be using such drugs across the globe. This also involves adjusting treatment options to suit local preferences and healthcare infrastructure. Furthermore, regulatory bodies across all nations have become more vigilant about safety profiles of drugs causing serotonin syndrome thus leading to tighter guidelines and monitoring.
Technological advancements are also important for shaping trends in serotonin syndrome market. Diagnostic accuracy as well as treatment planning has been improved through integration between artificial intelligence and data analytics. Also, telemedicine and remote monitoring have become an integral part that enables closer observation of individuals with this condition by physicians who intervene whenever necessary."


















Leave a Comment