Market Share
Retinal Biologics Market Share Analysis
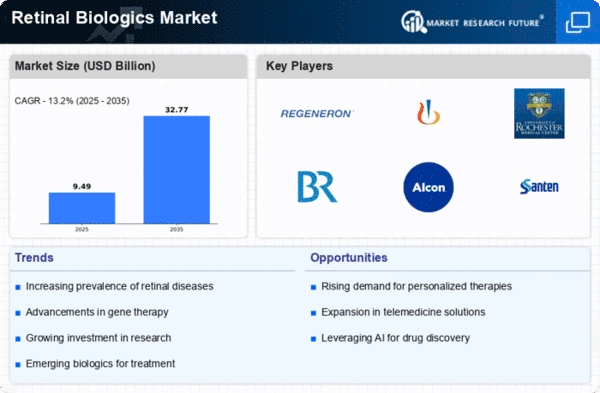
As firms vie to gain a foothold in the treatment of retinal conditions, the Pharmaceutical Retinal Biologics Market is currently experiencing strategic manoeuvres. One key approach that pharmaceutical firms use is product differentiation, with the aim of developing new and innovative biologic treatments for retinal disorders. They seek to differentiate themselves among healthcare professionals and patients looking for advanced solutions by making biologic products that have greater effectiveness, fewer side-effects or new mechanisms. Additionally, cost leadership is another important strategy in the Pharmaceutical Retinal Biologics Market. Through optimizing manufacturing processes, negotiating cost-effective sourcing of biological materials, and implementing efficient distribution channels that provide affordable biologic therapies. Because of this, these companies are creating drugs which can be bought easily by people who suffer from chronic retina ailments. To capture large market share and address broader health care issues for those affected by disease in the retina, they position themselves as leaders of retinal biologics at low costs. Innovation is pivotal when it comes to positioning market shares within the Pharmaceutical Retinal Biologics Market. The companies are investing significantly on research and development thereby introducing new biologics with improved efficacy against retina conditions. By being up-to date with scientific advancements the company positions itself as a leader thus earning trust from healthcare practitioners and patients too. Therefore, not only do innovative solutions meet patient’s needs dynamically but also enhance overall progress in relation to retinal disorders’ treatments. Topical collaborations and partnerships are getting more prominence within the Pharmaceutical Retinal Biologics Market. In order to improve their knowledge base and expand their geographic coverage, companies partner with Research Institutions (RI), Ophthalmology clinics (OC) or organizations focusing on retinal disorder (OF). Collaborative efforts encourage sharing of ideas enabling clinical trials in populations from different backgrounds therefore creating holistic approaches towards treating retinal diseases. By building collaborative networks; companies can strengthen their market presence while advancing retinal disorder care. Marketing and branding become significant in the market share positioning within the Pharmaceutical Retinal Biologics Market. In order to educate healthcare professionals about specific biologic therapies, raise awareness about retinal disorders and promote them, companies invest in targeted marketing campaigns. A strong brand presence not only improves a company’s reputation but also ensures that prescription decisions are made by healthcare providers who believe in it. Whether educational initiatives, digital marketing strategies or ophthalmology conferences; all these contribute to effective communication and interaction with customers. As firms recognise the significance of patient experience customer-centric approaches is gaining ground in the Pharmaceuticals Retinal Biologics Market. Positive experiences of patients are attributed to customizing biologic therapies according to their needs, facilitating easy administration procedures as well as offering patients support programs. They want to build loyalty among patients, secure positive reviews as well as gain huge market shares in this competitive market through focusing more on overall satisfaction and wellness of people living with problems of retina.


















Leave a Comment