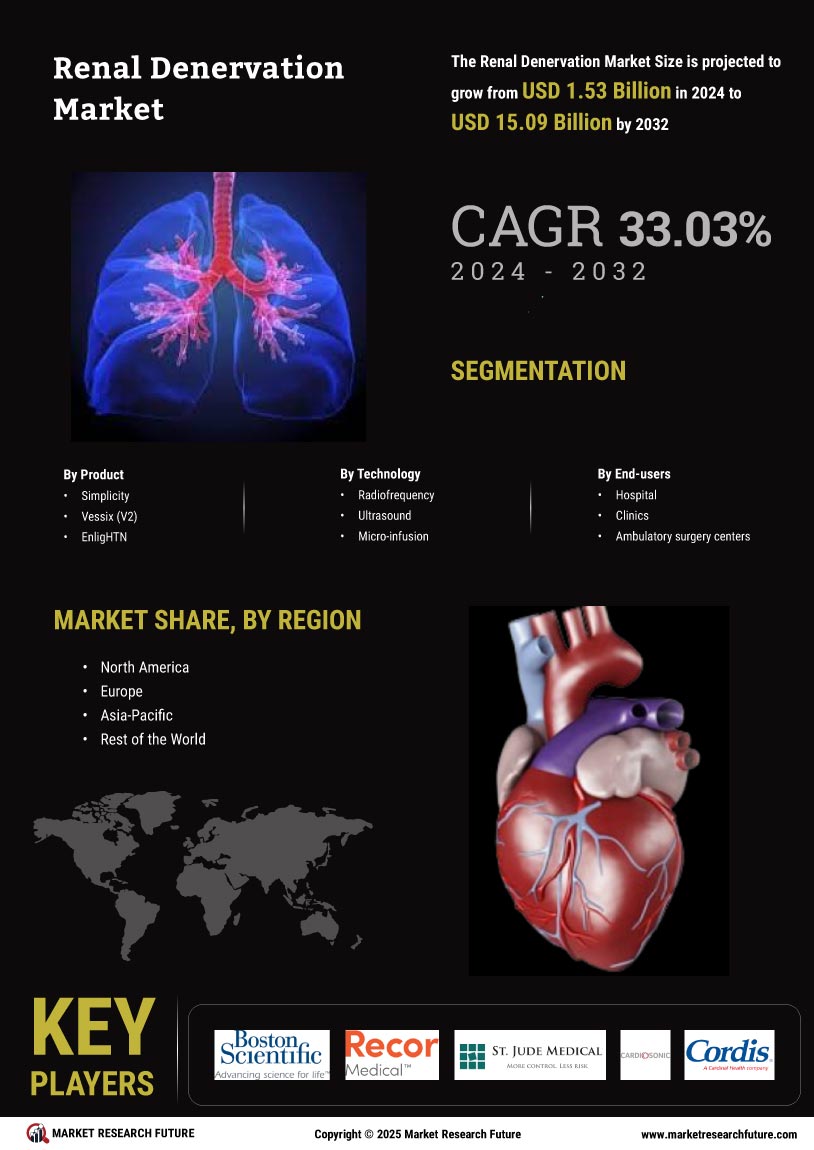
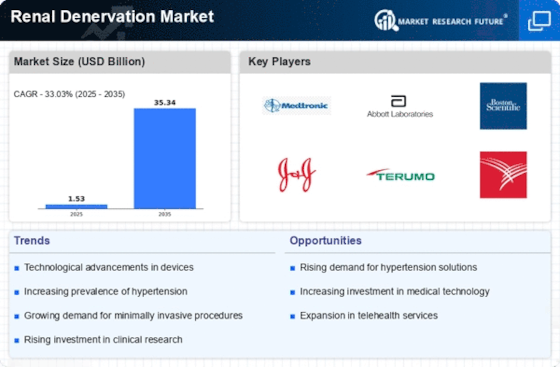
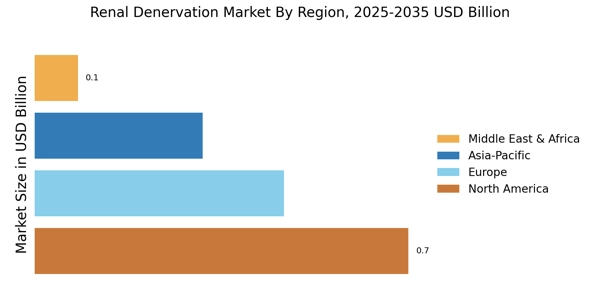
Growing Awareness and Education
The growing awareness and education surrounding hypertension and its management are pivotal drivers for the Renal Denervation Market. Healthcare professionals and patients alike are becoming more informed about the potential benefits of renal denervation as a treatment option. Educational initiatives, including workshops, seminars, and online resources, are helping to disseminate knowledge about the procedure's effectiveness and safety. This heightened awareness is likely to lead to increased patient referrals for renal denervation, as well as greater acceptance among healthcare providers. Consequently, the Renal Denervation Market may witness a rise in procedure volumes, as more patients seek out this innovative treatment for resistant hypertension.
Regulatory Support and Approvals
Regulatory support and approvals play a crucial role in shaping the Renal Denervation Market. Recent approvals from regulatory bodies for new renal denervation devices have instilled confidence among healthcare providers and patients. These approvals often follow rigorous clinical trials that demonstrate the safety and efficacy of the devices, paving the way for broader adoption in clinical settings. As more devices receive regulatory clearance, the market is likely to expand, with increased competition among manufacturers. This competitive landscape may lead to further innovations and improvements in renal denervation technologies, ultimately benefiting patients and healthcare systems. The Renal Denervation Market is thus poised for growth, driven by a favorable regulatory environment.
Increasing Healthcare Expenditure
The rise in healthcare expenditure across various regions is a significant driver for the Renal Denervation Market. As countries allocate more resources to healthcare, there is a growing emphasis on innovative treatment options for chronic conditions such as hypertension. Increased funding for medical technologies and procedures allows healthcare providers to invest in advanced renal denervation systems, which can lead to improved patient outcomes. Moreover, as healthcare systems recognize the long-term cost savings associated with effective hypertension management, the adoption of renal denervation is likely to increase. This trend suggests that the Renal Denervation Market will continue to thrive, supported by enhanced healthcare investments and a focus on improving patient care.
Rising Prevalence of Hypertension
The increasing prevalence of hypertension is a primary driver for the Renal Denervation Market. As hypertension affects a substantial portion of the adult population, estimated at around 1.13 billion individuals worldwide, the demand for effective treatment options is surging. Renal denervation, a minimally invasive procedure, offers a promising alternative for patients who do not respond well to traditional antihypertensive medications. This growing patient population is likely to propel the market forward, as healthcare providers seek innovative solutions to manage hypertension effectively. Furthermore, the Renal Denervation Market is expected to benefit from the rising awareness of hypertension-related complications, which may lead to increased screening and diagnosis rates, ultimately driving demand for renal denervation procedures.
Technological Innovations in Devices
Technological advancements in renal denervation devices are significantly influencing the Renal Denervation Market. Recent innovations, such as the development of catheter-based systems and improved energy delivery mechanisms, have enhanced the efficacy and safety of the procedure. For instance, the introduction of radiofrequency and ultrasound-based systems has shown promising results in clinical trials, leading to better patient outcomes. These advancements not only improve procedural success rates but also reduce recovery times, making renal denervation a more attractive option for both patients and healthcare providers. As these technologies continue to evolve, the Renal Denervation Market is likely to experience accelerated growth, driven by the increasing adoption of these advanced devices in clinical practice.