Market Growth Projections
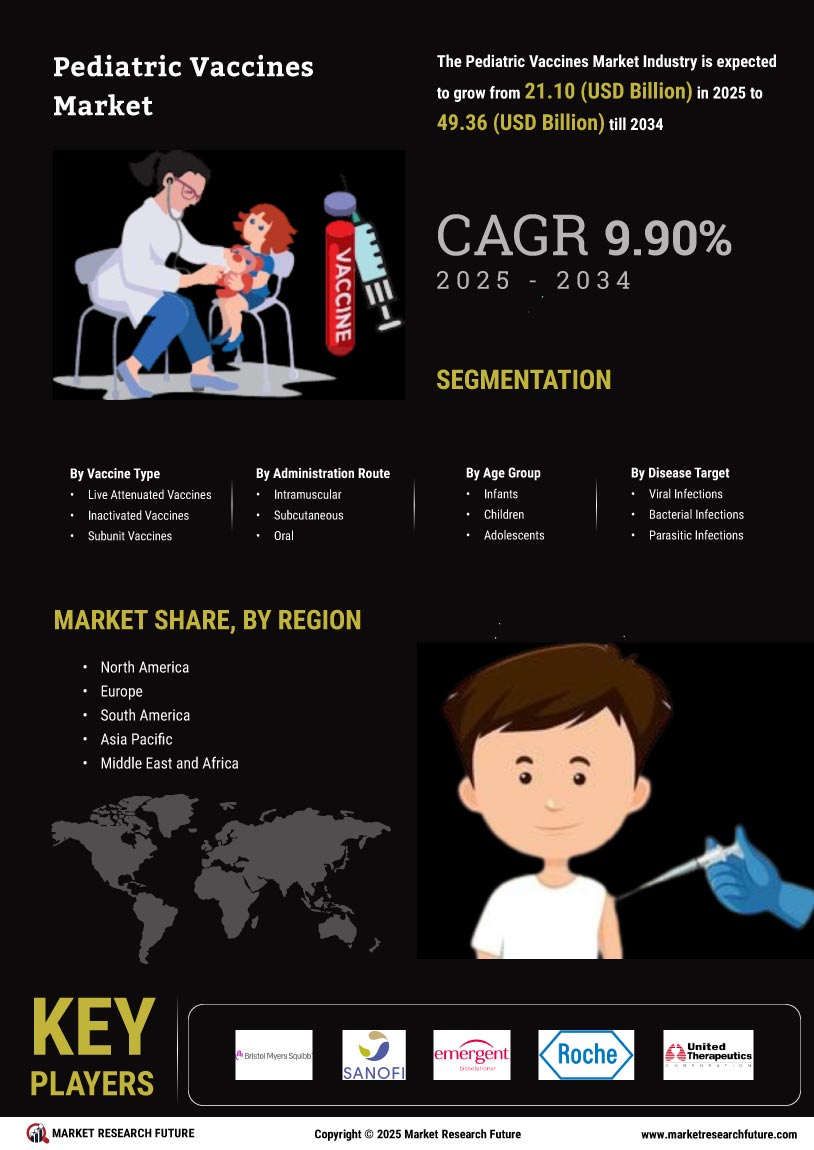
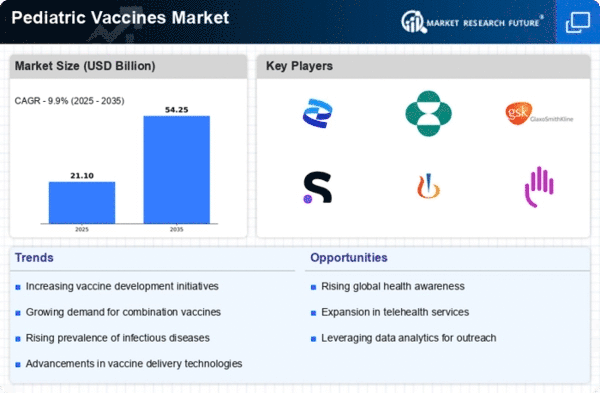
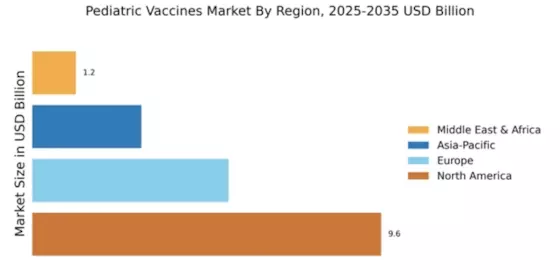
The Global Pediatric Vaccines Market Industry is poised for robust growth, with projections indicating a market value of 19.2 USD Billion in 2024 and an anticipated increase to 54.2 USD Billion by 2035. This growth trajectory suggests a compound annual growth rate of 9.9% from 2025 to 2035. Such figures reflect the increasing recognition of the importance of vaccination in public health and the ongoing efforts to improve vaccine access and coverage globally. The market's expansion is likely to be driven by a combination of factors, including rising disease incidence, government initiatives, and technological advancements.
Government Initiatives and Funding
Government initiatives play a crucial role in shaping the Global Pediatric Vaccines Market Industry. Many countries are implementing national immunization programs that provide free or subsidized vaccines to children. For example, the Global Alliance for Vaccines and Immunization (GAVI) collaborates with governments to enhance vaccine access in developing nations. Such initiatives not only increase vaccination coverage but also stimulate market growth by ensuring a steady demand for pediatric vaccines. As funding for immunization programs continues to rise, the market is expected to expand significantly, with projections indicating a value of 54.2 USD Billion by 2035.
Emerging Markets and Population Growth
Emerging markets are becoming increasingly important in the Global Pediatric Vaccines Market Industry. Countries in Asia, Africa, and Latin America are experiencing rapid population growth, leading to a larger cohort of children requiring vaccinations. This demographic shift presents a substantial opportunity for vaccine manufacturers to expand their reach. Additionally, as these regions develop economically, there is a growing emphasis on healthcare infrastructure, which supports vaccination programs. The combination of population growth and improved healthcare access is likely to drive significant demand for pediatric vaccines, further solidifying the market's expansion.
Increased Awareness of Vaccine Benefits
Public awareness regarding the benefits of vaccination is a driving force in the Global Pediatric Vaccines Market Industry. Campaigns aimed at educating parents about the importance of immunization have led to higher vaccination rates. For instance, initiatives by health organizations highlight the role of vaccines in preventing serious diseases and promoting overall child health. This increased awareness is particularly crucial in combating vaccine hesitancy, which can hinder immunization efforts. As more parents recognize the value of vaccines, the demand for pediatric vaccines is expected to rise, contributing to the market's growth trajectory.
Rising Incidence of Infectious Diseases
The Global Pediatric Vaccines Market Industry is experiencing growth due to the increasing incidence of infectious diseases among children. For instance, diseases such as measles and pertussis have seen a resurgence in various regions, prompting health authorities to advocate for vaccination. This trend is particularly evident in low- and middle-income countries, where vaccination rates have historically lagged. The World Health Organization emphasizes the importance of immunization in preventing outbreaks, which further drives demand for pediatric vaccines. As a result, the market is projected to reach 19.2 USD Billion in 2024, reflecting a heightened focus on child health and disease prevention.
Technological Advancements in Vaccine Development
Technological advancements are transforming the Global Pediatric Vaccines Market Industry by enabling the development of more effective and safer vaccines. Innovations such as mRNA technology and nanoparticle-based vaccines are being explored to enhance immunogenicity and reduce side effects. These advancements not only improve vaccine efficacy but also expedite the development process, allowing for quicker responses to emerging infectious diseases. As a result, the market is likely to benefit from increased investment in research and development, which could lead to a compound annual growth rate of 9.9% from 2025 to 2035, reflecting the potential for significant growth in the coming years.