Rising Incidence of Craniosynostosis
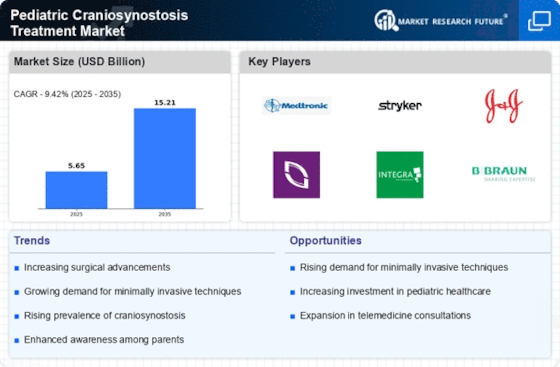
The Pediatric Craniosynostosis Treatment Market is experiencing growth due to the rising incidence of craniosynostosis among infants. Recent data indicates that craniosynostosis affects approximately 1 in 2,000 live births, leading to an increased demand for effective treatment options. As awareness of this condition grows, parents are more likely to seek medical intervention, thereby driving market expansion. The prevalence of this condition necessitates the establishment of specialized treatment protocols and facilities, which further contributes to the market's development. Additionally, the increasing number of healthcare professionals trained in pediatric craniofacial surgery enhances the capacity to address this condition, potentially leading to improved patient outcomes and satisfaction.
Advancements in Surgical Technologies
Technological innovations in surgical techniques are significantly influencing the Pediatric Craniosynostosis Treatment Market. Minimally invasive procedures, such as endoscopic strip craniectomy, have emerged as effective alternatives to traditional open surgeries. These advancements not only reduce recovery times but also minimize complications, making them appealing to both healthcare providers and parents. The integration of 3D imaging and modeling technologies allows for more precise surgical planning, which can enhance the overall success rates of procedures. As these technologies continue to evolve, they are likely to attract more patients seeking advanced treatment options, thereby propelling market growth. Furthermore, the development of new surgical instruments tailored for pediatric use is expected to further enhance the efficacy of craniosynostosis treatments.
Growing Parental Awareness and Education
Parental awareness regarding craniosynostosis is on the rise, which is positively impacting the Pediatric Craniosynostosis Treatment Market. Educational campaigns and resources provided by healthcare organizations are helping parents recognize the signs and symptoms of craniosynostosis earlier. This increased awareness leads to earlier diagnosis and intervention, which is critical for optimal treatment outcomes. As parents become more informed, they are more likely to seek specialized care for their children, thereby driving demand for treatment services. Furthermore, support groups and online communities are playing a vital role in disseminating information, fostering a proactive approach among parents. This trend is likely to continue, further enhancing the market's growth as more families seek timely and effective treatment options.
Emergence of Telemedicine in Pediatric Care
The emergence of telemedicine is transforming the Pediatric Craniosynostosis Treatment Market by providing greater access to specialized care. Telehealth services enable families in remote or underserved areas to consult with craniofacial specialists without the need for extensive travel. This accessibility is particularly beneficial for early diagnosis and follow-up care, which are essential for managing craniosynostosis effectively. As telemedicine technology continues to improve, it is expected that more families will utilize these services, thereby increasing the overall patient base for craniosynostosis treatments. Additionally, telemedicine can facilitate better communication between healthcare providers and families, ensuring that patients receive timely and appropriate care. This trend may lead to a more efficient healthcare delivery system, ultimately benefiting the Pediatric Craniosynostosis Treatment Market.
Increased Investment in Pediatric Healthcare
The Pediatric Craniosynostosis Treatment Market is benefiting from increased investment in pediatric healthcare. Governments and private organizations are recognizing the importance of addressing congenital conditions, leading to enhanced funding for research and treatment facilities. This financial support is crucial for developing innovative treatment methodologies and improving existing care standards. For instance, investments in specialized craniofacial centers have been shown to improve patient outcomes significantly. Additionally, the establishment of multidisciplinary teams that include pediatricians, neurosurgeons, and plastic surgeons is becoming more common, which enhances the quality of care provided to affected children. As funding continues to grow, it is anticipated that the market will expand, offering more comprehensive treatment options for families.