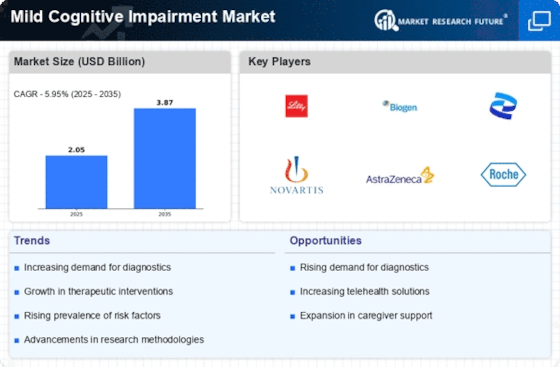
Top Industry Leaders in the Mild Cognitive Impairment Market

Eli Lilly receives Fast Track designation for donanemab in MCI: In December 2023, Lilly received FDA Fast Track designation for donanemab, an anti-amyloid antibody, for the treatment of early Alzheimer's disease and MCI due to Alzheimer's pathology. This signifies a potential acceleration in the drug's approval process.
Biogen announces plans for Phase 3 trials of aducanumab in MCI Despite aducanumab's controversial approval for Alzheimer's disease, Biogen is planning Phase 3 trials to assess its efficacy in MCI prevention. This could potentially expand the drug's reach earlier in the disease progression.
TauRx Therapeutics reports positive Phase 2a results for TauRX301 in progressive supranuclear palsy (PSP) While not directly targeting MCI, TauRx Therapeutics' positive Phase 2a results for TauRX301 offer hope for developing similar Tau-targeting therapies for MCI and other neurodegenerative diseases.
- Pfizer Inc. (US)
- Hoffman La Roche Ltd (Switzerland)
- Novartis AG (Switzerland)
- Accord UK Ltd (UK)
- Reddy’s laboratories Ltd (India)
- Hikma Pharmaceuticals PLC (UK)
- Sun Pharmaceuticals ltd (India)
- Johnson & Johnson Services, Inc. (US)
- Teva Pharmaceutical Industries Ltd., (Israel)
- Takeda Pharmaceutical Company Limited (US)