North America : Market Leader in Innovation
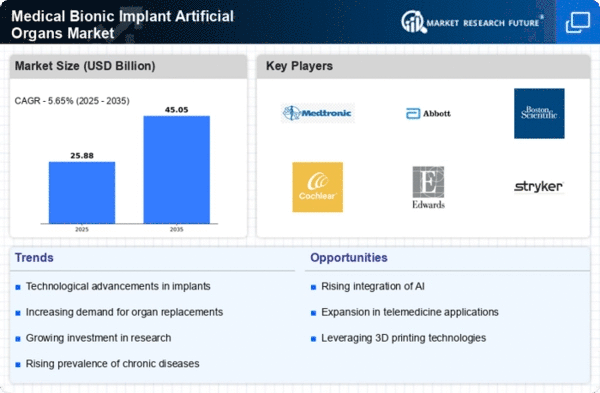
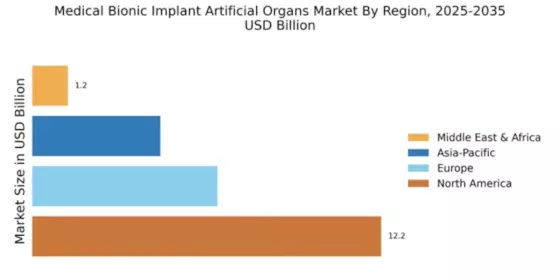
North America is poised to maintain its leadership in the Medical Bionic Implant Artificial Organs Market, holding a significant market share of 12.25 in 2024. The region's growth is driven by advanced healthcare infrastructure, increasing prevalence of chronic diseases, and a strong focus on research and development. Regulatory support from agencies like the FDA further catalyzes innovation, ensuring rapid approval of new technologies and devices.
The competitive landscape is robust, with key players such as Medtronic, Abbott Laboratories, and Boston Scientific leading the charge. The U.S. remains the largest market, benefiting from high healthcare expenditure and a growing aging population. Companies are increasingly investing in partnerships and collaborations to enhance product offerings and expand their market reach, solidifying North America's position as a hub for medical bionic innovations.
Europe : Emerging Market with Potential
Europe is witnessing a growing interest in the Medical Bionic Implant Artificial Organs Market, with a market size of 6.5 in 2024. Factors such as an aging population, rising healthcare costs, and advancements in technology are driving demand. Regulatory frameworks, including the EU Medical Device Regulation, are enhancing safety and efficacy standards, which in turn boosts consumer confidence and market growth.
Leading countries like Germany, France, and the UK are at the forefront of this market, supported by strong healthcare systems and innovative research initiatives. Key players such as B. Braun Melsungen AG and Cochlear Limited are actively expanding their presence. The competitive landscape is characterized by a mix of established firms and emerging startups, fostering a dynamic environment for innovation and collaboration.
Asia-Pacific : Rapidly Growing Market
Asia-Pacific is emerging as a rapidly growing market for Medical Bionic Implant Artificial Organs, with a market size of 4.5 in 2024. The region's growth is fueled by increasing healthcare investments, rising awareness of advanced medical technologies, and a growing population with chronic health conditions. Government initiatives aimed at improving healthcare access and affordability are also significant drivers of market expansion.
Countries like China, Japan, and India are leading the charge, with substantial investments in healthcare infrastructure and technology. The competitive landscape features both global giants and local players, creating a diverse market environment. Companies are focusing on innovation and localization strategies to cater to the unique needs of the region, enhancing their market presence and competitiveness.
Middle East and Africa : Emerging Market Opportunities
The Middle East and Africa region is gradually emerging in the Medical Bionic Implant Artificial Organs Market, with a market size of 1.25 in 2024. The growth is driven by increasing healthcare investments, rising awareness of advanced medical technologies, and a growing population with chronic health conditions. Government initiatives aimed at improving healthcare access and affordability are also significant drivers of market expansion.
Countries like South Africa and the UAE are leading the charge, with substantial investments in healthcare infrastructure and technology. The competitive landscape features both global giants and local players, creating a diverse market environment. Companies are focusing on innovation and localization strategies to cater to the unique needs of the region, enhancing their market presence and competitiveness.