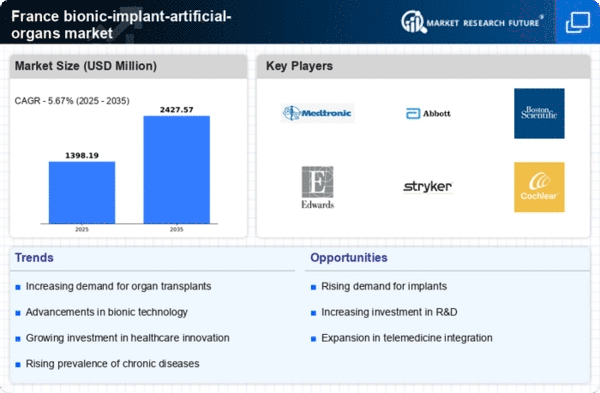
The France Medical Bionic Implant Artificial Organs Market presents a landscape characterized by significant innovation and rapid advancements in technology, catering to the needs of patients requiring life-enhancing solutions. The competitive environment in this market is influenced by an increasing prevalence of chronic diseases, a rising aging population, and heightened awareness regarding organ transplantation alternatives. This segment is marked by a continuous push for research and development initiatives focused on refining the performance, longevity, and biocompatibility of artificial organs.
Stakeholders are actively engaging in strategic partnerships, collaborations, and mergers to leverage complementary technologies and expand their market reach, thereby fostering a dynamic and competitive ecosystem.
Medtronic has established a formidable presence in the France Medical Bionic Implant Artificial Organs Market, driven by a robust portfolio of innovative products and industry recognition. The company's strengths lie in its extensive experience, strong research capabilities, and commitment to advancing healthcare technologies. By focusing on patient outcomes and technological enhancements, Medtronic has developed several successful medical devices that address critical health challenges. The company’s targeted marketing strategies, coupled with a deep understanding of the French healthcare system, enable it to effectively navigate the complex regulatory landscape.
Medtronic's ability to forge collaborations with healthcare professionals and institutions further solidifies its competitive standing, allowing it to remain at the forefront of developments in the field of bionic implants and artificial organ solutions.
Cochlear Limited, operating rigorously within the France Medical Bionic Implant Artificial Organs Market, specializes in designing, manufacturing, and distributing implantable hearing solutions. The company's flagship products include cochlear implants, which have gained traction for their ability to restore hearing to patients with severe-to-profound hearing loss. Cochlear Limited's strengths are reflected in its strong commitment to innovation and patient care, as well as its extensive clinical evidence supporting the efficacy of its products. The company actively extends its market presence in France through strategic mergers and acquisitions aimed at expanding its technological capabilities and enhancing customer access.
As a result, Cochlear Limited is well-positioned to capture growth opportunities within the French market while continuously delivering cutting-edge solutions and enhancing the quality of life for its patients.



















Leave a Comment