Growing Awareness and Advocacy
The increasing awareness and advocacy for lysosomal storage diseases are pivotal in shaping the Lysosomal Storage Diseases Therapeutics Market. Patient advocacy groups and non-profit organizations are actively working to educate the public and healthcare professionals about these conditions. This heightened awareness leads to earlier diagnosis and treatment, which is crucial for improving patient outcomes. Furthermore, advocacy efforts are driving funding for research and development, as well as influencing policy changes that support access to therapies. As awareness continues to grow, the demand for effective therapeutics is likely to increase, further propelling market expansion.
Advancements in Biopharmaceuticals
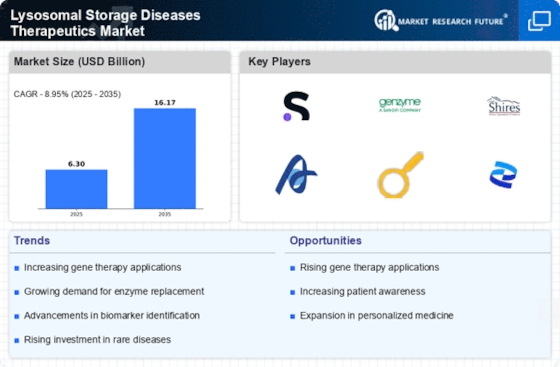
The rapid advancements in biopharmaceuticals are significantly influencing the Lysosomal Storage Diseases Therapeutics Market. Innovations in biologics, including monoclonal antibodies and enzyme replacement therapies, have transformed the treatment landscape for LSDs. For instance, the introduction of new enzyme replacement therapies has shown promising results in improving patient outcomes. The biopharmaceutical sector is projected to grow at a compound annual growth rate (CAGR) of over 8% in the coming years, indicating a robust pipeline of potential therapies for LSDs. These advancements not only enhance treatment efficacy but also expand the therapeutic options available to patients, thereby driving market growth.
Regulatory Support for Orphan Drugs
Regulatory bodies are providing enhanced support for the development of orphan drugs, which is positively impacting the Lysosomal Storage Diseases Therapeutics Market. Initiatives such as the Orphan Drug Act in various regions offer incentives, including tax credits and market exclusivity, to encourage pharmaceutical companies to develop treatments for rare diseases. This regulatory framework has led to a significant increase in the number of orphan drugs approved for LSDs, with over 20 therapies currently available. The favorable regulatory environment not only incentivizes research and development but also ensures that patients have access to essential therapies, thereby driving market growth.
Increased Investment in Rare Disease Research
The growing investment in research focused on rare diseases, including lysosomal storage diseases, is a crucial driver for the Lysosomal Storage Diseases Therapeutics Market. Governments and private organizations are increasingly allocating funds to support research initiatives aimed at understanding and treating these conditions. In recent years, funding for rare disease research has surged, with estimates indicating that over $1 billion is invested annually in the development of therapies for LSDs. This influx of capital fosters innovation and accelerates the development of new therapeutics, ultimately benefiting patients and expanding the market.
Rising Prevalence of Lysosomal Storage Diseases
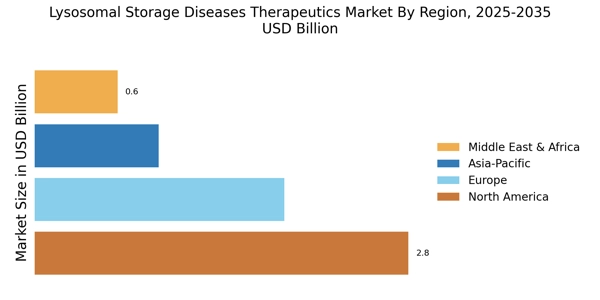
The increasing incidence of lysosomal storage diseases (LSDs) is a primary driver for the Lysosomal Storage Diseases Therapeutics Market. Recent estimates suggest that LSDs affect approximately 1 in 5,000 live births, with certain types, such as Gaucher disease and Fabry disease, being more prevalent in specific populations. This rising prevalence necessitates the development of effective therapeutics, thereby propelling market growth. As awareness of these diseases expands, healthcare providers are more likely to diagnose and treat affected individuals, further stimulating demand for innovative therapies. The growing patient population is expected to drive investments in research and development, leading to the introduction of novel treatment options in the market.