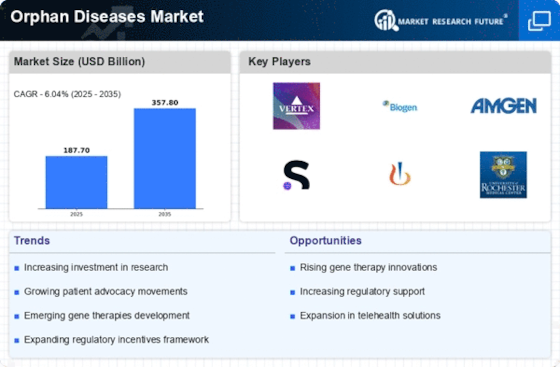
Increasing Prevalence of Orphan Diseases
The rising incidence of orphan diseases is a notable driver in the Orphan Diseases Market. As more diseases are classified as orphan, the demand for targeted therapies is likely to increase. According to recent estimates, approximately 7,000 distinct orphan diseases exist, affecting millions of individuals worldwide. This growing patient population creates a compelling need for innovative treatments, thereby attracting pharmaceutical companies to invest in the development of orphan drugs. The Orphan Diseases Market is expected to expand as more stakeholders recognize the potential for profitable ventures in addressing these unmet medical needs.
Rising Investment from Venture Capitalists
The influx of venture capital investment is significantly impacting the Orphan Diseases Market. Investors are increasingly recognizing the potential for high returns associated with orphan drug development, given the limited competition and the high unmet medical needs. Reports indicate that venture capital funding for biotech companies focusing on orphan diseases has surged in recent years, reflecting a growing confidence in the market's viability. This financial backing not only supports research and development efforts but also encourages startups to pursue innovative solutions for rare diseases. Consequently, the Orphan Diseases Market is poised for substantial growth as more capital flows into this sector.
Technological Advancements in Drug Development
Technological innovations are transforming the landscape of the Orphan Diseases Market. Advances in biotechnology, genomics, and data analytics are enabling researchers to identify and develop targeted therapies more efficiently. For example, the use of CRISPR technology has shown promise in gene editing for certain orphan diseases, potentially leading to groundbreaking treatments. Furthermore, the integration of artificial intelligence in drug discovery processes is streamlining the identification of viable drug candidates. These technological advancements not only enhance the speed of development but also improve the likelihood of successful outcomes, thereby driving growth in the Orphan Diseases Market.
Regulatory Incentives for Orphan Drug Development
Regulatory frameworks play a crucial role in shaping the Orphan Diseases Market. Governments and regulatory bodies have established various incentives, such as tax credits, grants, and extended market exclusivity, to encourage the development of orphan drugs. For instance, the Orphan Drug Act in the United States provides significant benefits to companies that develop treatments for rare diseases. These incentives not only lower the financial burden on developers but also expedite the approval process, thereby fostering a more dynamic market environment. As a result, the Orphan Diseases Market is likely to witness an influx of new therapies, enhancing treatment options for patients.
Collaboration Between Pharmaceutical Companies and Research Institutions
Collaborative efforts between pharmaceutical companies and research institutions are becoming increasingly prevalent in the Orphan Diseases Market. Such partnerships facilitate the sharing of resources, expertise, and data, which can accelerate the development of orphan drugs. By pooling knowledge and capabilities, stakeholders can tackle the complexities associated with rare diseases more effectively. This trend is evidenced by numerous joint ventures and alliances formed to address specific orphan diseases. As these collaborations continue to flourish, they are likely to enhance innovation and expedite the introduction of new therapies into the Orphan Diseases Market.