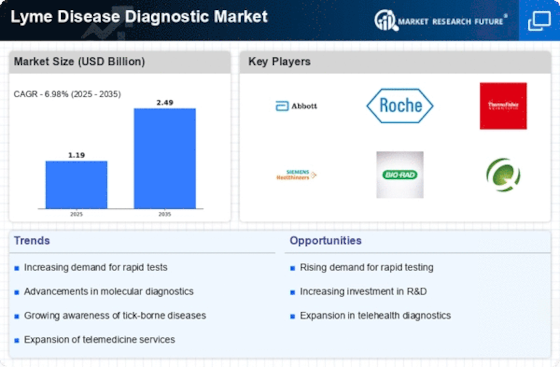
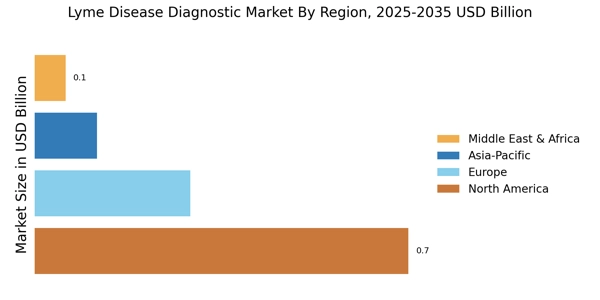
Rising Incidence of Lyme Disease
The increasing incidence of Lyme disease is a primary driver for the Lyme Disease Diagnostic Market. Reports indicate that the number of reported cases has been on the rise, with estimates suggesting that approximately 300,000 cases occur annually in the United States alone. This surge in cases necessitates improved diagnostic tools and methods, as timely and accurate diagnosis is crucial for effective treatment. The heightened prevalence of Lyme disease, particularly in endemic regions, underscores the need for innovative diagnostic solutions. As awareness grows among healthcare providers and patients, the demand for reliable diagnostic tests is likely to escalate, thereby propelling the Lyme Disease Diagnostic Market forward.
Growing Demand for Point-of-Care Testing
The growing demand for point-of-care testing (POCT) is emerging as a significant driver in the Lyme Disease Diagnostic Market. POCT offers the advantage of rapid diagnosis at the site of care, which is particularly beneficial in rural or underserved areas where access to laboratory facilities may be limited. The convenience and speed of POCT can lead to timely treatment decisions, improving patient outcomes. As healthcare providers seek to enhance the efficiency of care delivery, the adoption of point-of-care diagnostic tests for Lyme disease is likely to increase. This trend may stimulate further innovation and investment in the Lyme Disease Diagnostic Market, as stakeholders aim to meet the evolving needs of patients and healthcare systems.
Increased Public Awareness and Education
Increased public awareness and education regarding Lyme disease are pivotal in driving the Lyme Disease Diagnostic Market. Campaigns aimed at educating the public about the symptoms, transmission, and prevention of Lyme disease have led to a greater understanding of the condition. This heightened awareness encourages individuals to seek medical attention sooner, resulting in a higher demand for diagnostic testing. Furthermore, educational initiatives targeting healthcare professionals ensure that they are equipped with the knowledge to recognize and diagnose Lyme disease effectively. As awareness continues to grow, the Lyme Disease Diagnostic Market is likely to benefit from an uptick in testing and diagnostic services.
Regulatory Support and Funding Initiatives
Regulatory support and funding initiatives play a crucial role in shaping the Lyme Disease Diagnostic Market. Government agencies and health organizations are increasingly recognizing the need for improved diagnostic capabilities for Lyme disease. Funding for research and development of new diagnostic tests is on the rise, which may lead to the introduction of more effective and accessible diagnostic solutions. Additionally, regulatory bodies are streamlining the approval processes for new diagnostic technologies, facilitating quicker market entry. This supportive environment fosters innovation and encourages investment in the Lyme Disease Diagnostic Market, ultimately enhancing the availability of diagnostic tools for healthcare providers.
Technological Innovations in Diagnostic Tools
Technological advancements in diagnostic tools are significantly influencing the Lyme Disease Diagnostic Market. Innovations such as polymerase chain reaction (PCR) testing and advanced serological assays have enhanced the accuracy and speed of Lyme disease diagnosis. These technologies allow for the detection of the pathogen at earlier stages, which is critical for effective treatment. The market is witnessing a shift towards more sophisticated diagnostic solutions that can provide rapid results, thus improving patient outcomes. As healthcare systems increasingly adopt these advanced technologies, the Lyme Disease Diagnostic Market is expected to experience substantial growth, driven by the demand for more efficient and reliable diagnostic methods.