-
'TABLE OF CONTENTS
-
EXECUTIVE SUMMARY
-
MARKET INTRODUCTION
-
Definition
-
Scope of the Study
- Research Objective
-
Assumptions
-
Limitations
-
RESEARCH METHODOLOGY
-
Overview
-
Data Mining
-
Secondary Research
-
Primary Research
-
Primary Interviews and Information Gathering Process
-
Breakdown of Primary
-
Respondents
-
Forecasting Model
-
Market Size Estimation
-
Bottom-Up Approach
-
Top-Down Approach
-
Data Triangulation
-
Validation
-
MARKET DYNAMICS
-
Overview
-
Drivers
-
Restraints
-
Opportunities
-
MARKET FACTOR ANALYSIS
-
Value Chain Analysis
-
Porter’s Five Forces Analysis
- Bargaining
-
Power of Suppliers
-
Bargaining Power of Buyers
- Threat of
-
New Entrants
-
Threat of Substitutes
- Intensity of Rivalry
-
COVID-19 Impact Analysis
- Market Impact Analysis
-
Regional Impact
-
Opportunity and Threat Analysis
-
GLOBAL LAMBERT-EATON
-
MYASTHENIC SYNDROME TREATMENT MARKET, BY TYPE
-
Overview
-
Paraneoplastic
-
Idiopathic
-
GLOBAL LAMBERT-EATON MYASTHENIC SYNDROME TREATMENT MARKET,
-
BY TREATMENT TYPE
-
Overview
-
Immune Therapy
-
Medication
-
Plasmapheresis
-
Others
-
GLOBAL LAMBERT-EATON MYASTHENIC SYNDROME
-
TREATMENT MARKET, BY DRUGS
-
Overview
-
Cholinesterase Inhibitor
-
Potassium Channel Blockers
-
Intravenous Immunoglobulins
-
Others
-
GLOBAL LAMBERT-EATON MYASTHENIC SYNDROME TREATMENT MARKET, BY END
-
USER
-
Overview
-
Hospitals
-
Specialty Clinics
-
Homecare
-
GLOBAL LAMBERT-EATON MYASTHENIC SYNDROME TREATMENT MARKET, BY
-
REGION
-
Overview
-
North America
- US
-
Canada
-
Europe
- Germany
- France
- UK
- Italy
- Spain
- Rest of Europe
-
Asia-Pacific
- China
- India
- Japan
- South Korea
- Australia
- Rest of Asia-Pacific
-
Rest of the World
- Middle East
- Africa
- Latin America
-
COMPETITIVE LANDSCAPE
-
Overview
-
Competitive Analysis
-
Market
-
Share Analysis
-
Major Growth Strategy in the Global Lambert-Eaton Myasthenic
-
Syndrome Treatment Market,
-
Competitive Benchmarking
-
Leading
-
Players in Terms of Number of Developments in the Global Lambert-Eaton Myasthenic
-
Syndrome Treatment Market,
-
Key developments and Growth Strategies
- New Product Launch/Service Deployment
- Merger & Acquisitions
- Joint Ventures
-
Major Players Financial Matrix
-
Sales & Operating Income, 2023
-
Major Players R&D Expenditure.
-
COMPANY PROFILES
-
Catalyst Pharmaceuticals, Inc.
-
Company Overview
-
Financial Overview
- Products Offered
- Key Developments
- SWOT Analysis
- Key Strategies
-
Grifols, S.A.
- Company Overview
- Financial Overview
- Products Offered
- Key Developments
- SWOT Analysis
- Key Strategies
-
Alexion Pharmaceuticals, Inc.
- Company
-
Overview
-
Financial Overview
- Products Offered
-
Key Developments
-
SWOT Analysis
- Key Strategies
-
Argenx SE
-
Company Overview
- Financial Overview
-
Products Offered
-
Key Developments
- SWOT Analysis
-
Key Strategies
-
Immunovant, Inc.
- Company Overview
-
Financial Overview
-
Products Offered
- Key Developments
- SWOT Analysis
- Key Strategies
-
Ra Pharmaceuticals,
-
Inc.
-
Company Overview
- Financial Overview
-
Products Offered
-
Key Developments
- SWOT Analysis
-
Key Strategies
-
Bausch Health Companies Inc.
- Company Overview
- Financial Overview
- Products Offered
- Key Developments
- SWOT Analysis
- Key Strategies
-
JACOBUS PHARMACEUTICAL
-
COMPANY, INC.
-
Company Overview
- Financial Overview
-
Products Offered
-
Key Developments
- SWOT Analysis
-
Key Strategies
-
Takeda Pharmaceutical Company Limited
- Company
-
Overview
-
Financial Overview
- Products Offered
-
Key Developments
-
SWOT Analysis
- Key Strategies
-
Prestige Biopharma Limited
-
Company Overview
- Financial
-
Overview
-
Products Offered
- Key Developments
-
SWOT Analysis
-
Key Strategies
-
APPENDIX
-
References
-
Related Reports
-
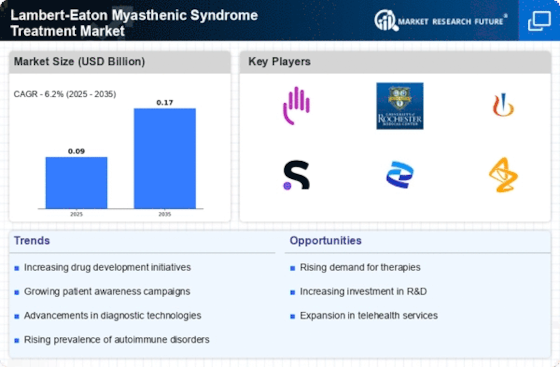
MYASTHENIC SYNDROME TREATMENT MARKET, SYNOPSIS, 2019-2032
-
MYASTHENIC SYNDROME TREATMENT MARKET, ESTIMATES & FORECAST, 2019-2032 (USD BILLION)
-
(USD BILLION)
-
BY TREATMENT TYPE, 2019-2032 (USD BILLION)
-
SYNDROME TREATMENT MARKET, BY DRUGS, 2019-2032 (USD BILLION)
-
LAMBERT-EATON MYASTHENIC SYNDROME TREATMENT MARKET, BY END USER, 2019-2032 (USD
-
BILLION)
-
MARKET, BY TYPE, 2019-2032 (USD BILLION)
-
MYASTHENIC SYNDROME TREATMENT MARKET, BY TREATMENT TYPE, 2019-2032 (USD BILLION)
-
BY DRUGS, 2019-2032 (USD BILLION)
-
SYNDROME TREATMENT MARKET, BY END USER, 2019-2032 (USD BILLION)
-
LAMBERT-EATON MYASTHENIC SYNDROME TREATMENT MARKET, BY TYPE, 2019-2032 (USD BILLION)
-
TYPE, 2019-2032 (USD BILLION)
-
TREATMENT MARKET, BY DRUGS, 2019-2032 (USD BILLION)
-
MYASTHENIC SYNDROME TREATMENT MARKET, BY END USER, 2019-2032 (USD BILLION)
-
CANADA: LAMBERT-EATON MYASTHENIC SYNDROME TREATMENT MARKET, BY TYPE, 2019-2032
-
(USD BILLION)
-
MARKET, BY TREATMENT TYPE, 2019-2032 (USD BILLION)
-
MYASTHENIC SYNDROME TREATMENT MARKET, BY DRUGS, 2019-2032 (USD BILLION)
-
CANADA: LAMBERT-EATON MYASTHENIC SYNDROME TREATMENT MARKET, BY END USER, 2019-2032
-
(USD BILLION)
-
MARKET, BY TYPE, 2019-2032 (USD BILLION)
-
SYNDROME TREATMENT MARKET, BY TREATMENT TYPE, 2019-2032 (USD BILLION)
-
EUROPE: LAMBERT-EATON MYASTHENIC SYNDROME TREATMENT MARKET, BY DRUGS, 2019-2032
-
(USD BILLION)
-
MARKET, BY END USER, 2019-2032 (USD BILLION)
-
MYASTHENIC SYNDROME TREATMENT MARKET, BY TYPE, 2019-2032 (USD BILLION)
-
GERMANY: LAMBERT-EATON MYASTHENIC SYNDROME TREATMENT MARKET, BY TREATMENT TYPE,
-
2032 (USD BILLION)
-
TREATMENT MARKET, BY DRUGS, 2019-2032 (USD BILLION)
-
MYASTHENIC SYNDROME TREATMENT MARKET, BY END USER, 2019-2032 (USD BILLION)
-
FRANCE: LAMBERT-EATON MYASTHENIC SYNDROME TREATMENT MARKET, BY TYPE, 2019-2032
-
(USD BILLION)
-
MARKET, BY TREATMENT TYPE, 2019-2032 (USD BILLION)
-
MYASTHENIC SYNDROME TREATMENT MARKET, BY DRUGS, 2019-2032 (USD BILLION)
-
FRANCE: LAMBERT-EATON MYASTHENIC SYNDROME TREATMENT MARKET, BY END USER, 2019-2032
-
(USD BILLION)
-
BY TYPE, 2019-2032 (USD BILLION)
-
TREATMENT MARKET, BY TREATMENT TYPE, 2019-2032 (USD BILLION)
-
LAMBERT-EATON MYASTHENIC SYNDROME TREATMENT MARKET, BY DRUGS, 2019-2032 (USD BILLION)
-
2032 (USD BILLION)
-
MARKET, BY TYPE, 2019-2032 (USD BILLION)
-
SYNDROME TREATMENT MARKET, BY TREATMENT TYPE, 2019-2032 (USD BILLION)
-
SPAIN: LAMBERT-EATON MYASTHENIC SYNDROME TREATMENT MARKET, BY DRUGS, 2019-2032
-
(USD BILLION)
-
BY END USER, 2019-2032 (USD BILLION)
-
SYNDROME TREATMENT MARKET, BY TYPE, 2019-2032 (USD BILLION)
-
MYASTHENIC SYNDROME TREATMENT MARKET, BY TREATMENT TYPE, 2019-2032 (USD BILLION)
-
(USD BILLION)
-
BY END USER, 2019-2032 (USD BILLION)
-
MYASTHENIC SYNDROME TREATMENT MARKET, BY TYPE, 2019-2032 (USD BILLION)
-
REST OF EUROPE: LAMBERT-EATON MYASTHENIC SYNDROME TREATMENT MARKET, BY TREATMENT
-
TYPE, 2019-2032 (USD BILLION)
-
SYNDROME TREATMENT MARKET, BY DRUGS, 2019-2032 (USD BILLION)
-
OF EUROPE: LAMBERT-EATON MYASTHENIC SYNDROME TREATMENT MARKET, BY END USER, 2019-2032
-
(USD BILLION)
-
MARKET, BY TYPE, 2019-2032 (USD BILLION)
-
MYASTHENIC SYNDROME TREATMENT MARKET, BY TREATMENT TYPE, 2019-2032 (USD BILLION)
-
BY DRUGS, 2019-2032 (USD BILLION)
-
SYNDROME TREATMENT MARKET, BY END USER, 2019-2032 (USD BILLION)
-
LAMBERT-EATON MYASTHENIC SYNDROME TREATMENT MARKET, BY TYPE, 2019-2032 (USD BILLION)
-
TYPE, 2019-2032 (USD BILLION)
-
TREATMENT MARKET, BY DRUGS, 2019-2032 (USD BILLION)
-
MYASTHENIC SYNDROME TREATMENT MARKET, BY END USER, 2019-2032 (USD BILLION)
-
CHINA: LAMBERT-EATON MYASTHENIC SYNDROME TREATMENT MARKET, BY TYPE, 2019-2032
-
(USD BILLION)
-
BY TREATMENT TYPE, 2019-2032 (USD BILLION)
-
SYNDROME TREATMENT MARKET, BY DRUGS, 2019-2032 (USD BILLION)
-
LAMBERT-EATON MYASTHENIC SYNDROME TREATMENT MARKET, BY END USER, 2019-2032 (USD
-
BILLION)
-
BY TYPE, 2019-2032 (USD BILLION)
-
SYNDROME TREATMENT MARKET, BY TREATMENT TYPE, 2019-2032 (USD BILLION)
-
INDIA: LAMBERT-EATON MYASTHENIC SYNDROME TREATMENT MARKET, BY DRUGS, 2019-2032
-
(USD BILLION)
-
MARKET, BY END USER, 2019-2032 (USD BILLION)
-
MYASTHENIC SYNDROME TREATMENT MARKET, BY TYPE, 2019-2032 (USD BILLION)
-
AUSTRALIA: LAMBERT-EATON MYASTHENIC SYNDROME TREATMENT MARKET, BY TREATMENT TYPE,
-
2032 (USD BILLION)
-
TREATMENT MARKET, BY DRUGS, 2019-2032 (USD BILLION)
-
MYASTHENIC SYNDROME TREATMENT MARKET, BY END USER, 2019-2032 (USD BILLION)
-
SOUTH KOREA: LAMBERT-EATON MYASTHENIC SYNDROME TREATMENT MARKET, BY TYPE, 2019-2032
-
(USD BILLION)
-
MARKET, BY TREATMENT TYPE, 2019-2032 (USD BILLION)
-
MYASTHENIC SYNDROME TREATMENT MARKET, BY DRUGS, 2019-2032 (USD BILLION)
-
SOUTH KOREA: LAMBERT-EATON MYASTHENIC SYNDROME TREATMENT MARKET, BY END USER,
-
2032 (USD BILLION)
-
SYNDROME TREATMENT MARKET, BY TYPE, 2019-2032 (USD BILLION)
-
OF ASIA-PACIFIC: LAMBERT-EATON MYASTHENIC SYNDROME TREATMENT MARKET, BY TREATMENT
-
TYPE, 2019-2032 (USD BILLION)
-
MYASTHENIC SYNDROME TREATMENT MARKET, BY DRUGS, 2019-2032 (USD BILLION)
-
REST OF ASIA-PACIFIC: LAMBERT-EATON MYASTHENIC SYNDROME TREATMENT MARKET, BY
-
END USER, 2019-2032 (USD BILLION)
-
MYASTHENIC SYNDROME TREATMENT MARKET, BY TYPE, 2019-2032 (USD BILLION)
-
REST OF THE WORLD: LAMBERT-EATON MYASTHENIC SYNDROME TREATMENT MARKET, BY TREATMENT
-
TYPE, 2019-2032 (USD BILLION)
-
SYNDROME TREATMENT MARKET, BY DRUGS, 2019-2032 (USD BILLION)
-
OF THE WORLD: LAMBERT-EATON MYASTHENIC SYNDROME TREATMENT MARKET, BY END USER, 2019-2032
-
(USD BILLION)
-
MARKET, BY TYPE, 2019-2032 (USD BILLION)
-
MYASTHENIC SYNDROME TREATMENT MARKET, BY TREATMENT TYPE, 2019-2032 (USD BILLION)
-
2032 (USD BILLION)
-
TREATMENT MARKET, BY END USER, 2019-2032 (USD BILLION)
-
MYASTHENIC SYNDROME TREATMENT MARKET, BY TYPE, 2019-2032 (USD BILLION)
-
AFRICA: LAMBERT-EATON MYASTHENIC SYNDROME TREATMENT MARKET, BY TREATMENT TYPE,
-
2032 (USD BILLION)
-
TREATMENT MARKET, BY DRUGS, 2019-2032 (USD BILLION)
-
MYASTHENIC SYNDROME TREATMENT MARKET, BY END USER, 2019-2032 (USD BILLION)
-
LATIN AMERICA: LAMBERT-EATON MYASTHENIC SYNDROME TREATMENT MARKET, BY TYPE, 2019-2032
-
(USD BILLION)
-
MARKET, BY TREATMENT TYPE, 2019-2032 (USD BILLION)
-
LAMBERT-EATON MYASTHENIC SYNDROME TREATMENT MARKET, BY DRUGS, 2019-2032 (USD BILLION)
-
END USER, 2019-2032 (USD BILLION)
-
MARKET
-
TREATMENT MARKET
-
MARKET, SHARE (%), BY TYPE, 2023
-
TREATMENT MARKET, SHARE (%), BY TREATMENT TYPE, 2023
-
MYASTHENIC SYNDROME TREATMENT MARKET, SHARE (%), BY DRUGS, 2023
-
LAMBERT-EATON MYASTHENIC SYNDROME TREATMENT MARKET, SHARE (%), BY END USER, 2023
-
(%), BY END USER, 2023
-
MARKET, SHARE (%), BY DRUGSRS, 2023
-
SYNDROME TREATMENT MARKET, SHARE (%), BY END USER, 2023
-
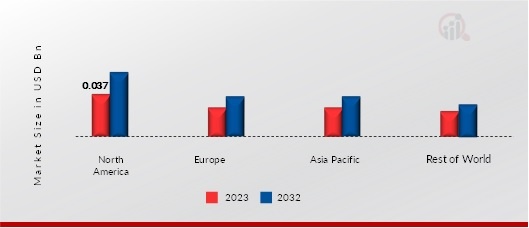
MYASTHENIC SYNDROME TREATMENT MARKET, SHARE (%), BY REGION, 2023
-
NORTH AMERICA: LAMBERT-EATON MYASTHENIC SYNDROME TREATMENT MARKET, SHARE (%), BY
-
REGION, 2023
-
MARKET, SHARE (%), BY REGION, 2023
-
SYNDROME TREATMENT MARKET, SHARE (%), BY REGION, 2023
-
WORLD: LAMBERT-EATON MYASTHENIC SYNDROME TREATMENT MARKET, SHARE (%), BY REGION,
-
COMPANY SHARE ANALYSIS, 2023 (%)
-
FINANCIAL OVERVIEW SNAPSHOT
-
ANALYSIS
-
GRIFOLS, S.A.: SWOT ANALYSIS
-
OVERVIEW SNAPSHOT
-
ANALYSIS
-
IMMUNOVANT, INC..: SWOT ANALYSIS
-
OVERVIEW SNAPSHOT
-
BAUSCH HEALTH COMPANIES INC.: FINANCIAL OVERVIEW SNAPSHOT
-
HEALTH COMPANIES INC.: SWOT ANALYSIS
-
INC.: FINANCIAL OVERVIEW SNAPSHOT
-
INC.: SWOT ANALYSIS
-
OVERVIEW SNAPSHOT
-
PRESTIGE BIOPHARMA LIMITED: SWOT ANALYSIS'