Supportive Government Policies
Government initiatives aimed at improving healthcare access and treatment options are positively impacting the Global Klinefelter Syndrome Treatment Market Industry. Policies that promote research funding, subsidized treatments, and public health campaigns are essential in facilitating better healthcare outcomes for individuals with Klinefelter Syndrome. Such supportive measures not only enhance the availability of treatment options but also encourage healthcare providers to adopt best practices in managing the syndrome. As governments continue to prioritize rare genetic conditions, the market is likely to see sustained growth, aligning with the projected increase in market value.
Advancements in Treatment Options
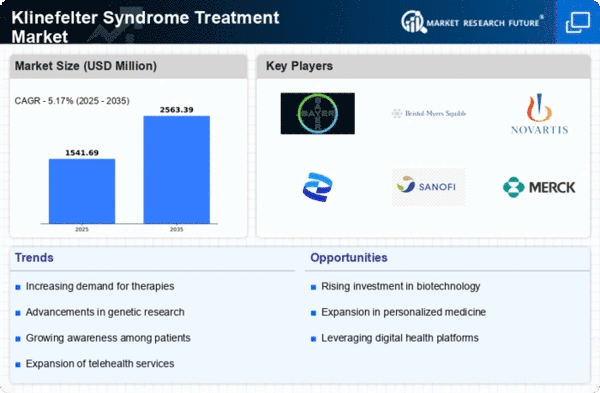
Innovations in treatment methodologies are driving the Global Klinefelter Syndrome Treatment Market Industry forward. Recent advancements include hormone replacement therapies and assisted reproductive technologies, which have shown promising results in managing symptoms and improving quality of life for affected individuals. These developments not only enhance patient care but also attract investment into research and development. As new therapies emerge, the market is likely to witness a significant increase in demand, with projections indicating a growth to 2.63 USD Billion by 2035. This growth underscores the importance of continuous innovation in treatment options.
Integration of Telemedicine in Treatment
The integration of telemedicine into the management of Klinefelter Syndrome is transforming the Global Klinefelter Syndrome Treatment Market Industry. Telehealth services provide patients with easier access to specialists, particularly in remote areas, thereby improving treatment adherence and follow-up care. This shift towards digital health solutions is particularly relevant in the context of ongoing advancements in technology. As telemedicine becomes more widely accepted, it is expected to enhance patient engagement and satisfaction, ultimately contributing to market growth. The convenience and accessibility offered by telemedicine may play a crucial role in shaping future treatment paradigms.
Rising Incidence of Klinefelter Syndrome
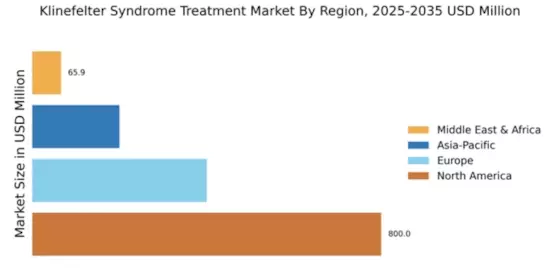
The Global Klinefelter Syndrome Treatment Market Industry is influenced by the rising incidence of Klinefelter Syndrome, which is estimated to affect approximately 1 in 600 males. This increasing prevalence is prompting healthcare systems to prioritize the development of effective treatment strategies. As more individuals are diagnosed, the need for specialized care and management solutions becomes evident. Consequently, this trend is expected to drive market growth significantly. The anticipated compound annual growth rate of 16.94% from 2025 to 2035 reflects the urgency to address the needs of this growing patient population.
Increasing Awareness of Klinefelter Syndrome
The Global Klinefelter Syndrome Treatment Market Industry is experiencing a surge in awareness regarding Klinefelter Syndrome, particularly among healthcare professionals and patients. This heightened awareness is crucial as it leads to earlier diagnosis and intervention, which can significantly improve patient outcomes. Educational initiatives and campaigns by health organizations are playing a pivotal role in disseminating information about the syndrome. As awareness grows, it is anticipated that the demand for treatment options will increase, contributing to the market's expansion. The industry is projected to reach 0.47 USD Billion in 2024, reflecting the impact of these awareness efforts.