Regulatory Support for Orphan Drugs
Regulatory frameworks supporting orphan drugs are playing a crucial role in the Hunter Syndrome Treatment Market. Governments and regulatory bodies are implementing policies that incentivize the development of treatments for rare diseases, including Hunter Syndrome. These incentives often include tax breaks, extended market exclusivity, and expedited review processes, which encourage pharmaceutical companies to invest in research and development. As a result, the number of orphan drugs entering the market is on the rise, providing patients with more therapeutic options. Market data suggests that the regulatory environment is becoming increasingly favorable for the approval of innovative therapies, which is likely to enhance the growth prospects of the Hunter Syndrome Treatment Market. This supportive landscape is essential for addressing the needs of patients with Hunter Syndrome.
Rising Awareness and Advocacy Efforts
The increasing awareness and advocacy efforts surrounding Hunter Syndrome are significantly influencing the Hunter Syndrome Treatment Market. Patient advocacy groups and healthcare organizations are actively working to educate the public and healthcare professionals about the disorder, its symptoms, and available treatment options. This heightened awareness is leading to earlier diagnoses and improved access to care for affected individuals. Furthermore, advocacy initiatives are fostering collaboration among stakeholders, including researchers, clinicians, and policymakers, to address the challenges faced by patients. Market trends indicate that as awareness grows, so does the demand for effective treatments, thereby driving the expansion of the Hunter Syndrome Treatment Market. The collective efforts of advocacy groups are likely to continue shaping the treatment landscape for Hunter Syndrome.
Increasing Prevalence of Hunter Syndrome
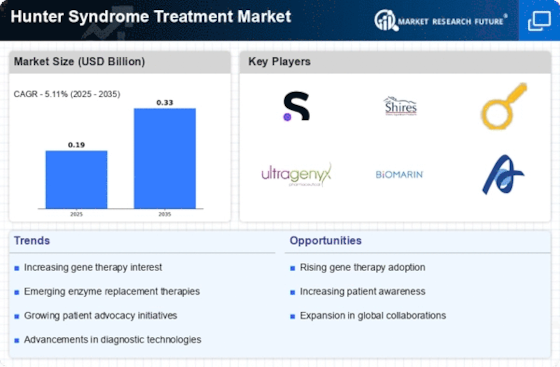
The rising incidence of Hunter Syndrome is a pivotal driver for the Hunter Syndrome Treatment Market. Recent estimates suggest that the disorder affects approximately 1 in 100,000 live births, leading to a growing patient population requiring effective treatment options. As awareness of the disease increases, more individuals are being diagnosed, which in turn fuels demand for innovative therapies. The need for specialized care and management strategies is becoming increasingly apparent, prompting healthcare providers to seek advanced treatment modalities. This trend is likely to continue, as ongoing research and development efforts aim to address the unmet needs of patients suffering from this rare genetic disorder. Consequently, the increasing prevalence of Hunter Syndrome is expected to significantly impact the growth trajectory of the Hunter Syndrome Treatment Market.
Advancements in Enzyme Replacement Therapy
Recent advancements in enzyme replacement therapy (ERT) are transforming the landscape of the Hunter Syndrome Treatment Market. ERT has emerged as a cornerstone of treatment, providing patients with the missing enzyme necessary for metabolic function. The introduction of new ERT products has shown promising results in clinical trials, demonstrating improved efficacy and safety profiles. Market data indicates that the ERT segment is projected to witness substantial growth, driven by the increasing adoption of these therapies among healthcare providers. Furthermore, the development of next-generation ERT formulations may enhance patient compliance and treatment outcomes. As a result, the advancements in ERT are likely to play a crucial role in shaping the future of the Hunter Syndrome Treatment Market.
Growing Investment in Rare Disease Research
The surge in investment directed towards rare disease research is a significant catalyst for the Hunter Syndrome Treatment Market. Pharmaceutical companies and research institutions are increasingly allocating resources to develop novel therapies for rare genetic disorders, including Hunter Syndrome. This trend is evidenced by the rising number of clinical trials and research initiatives focused on innovative treatment approaches. Market analysis reveals that funding for rare disease research has seen a marked increase, with public and private sectors collaborating to expedite drug development. This influx of investment not only accelerates the discovery of new treatment options but also enhances the overall understanding of Hunter Syndrome, thereby fostering a more robust treatment landscape. Consequently, the growing investment in rare disease research is poised to drive advancements within the Hunter Syndrome Treatment Market.