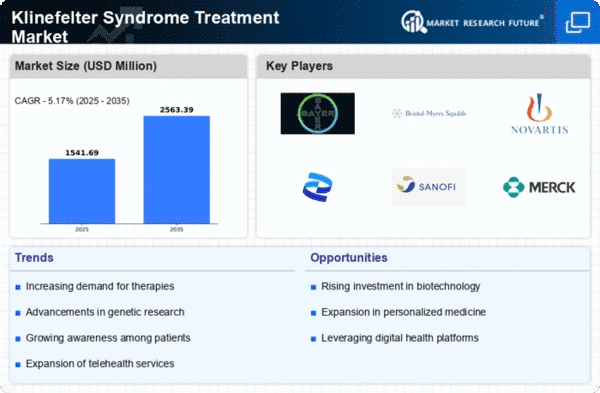
Market Trends
Key Emerging Trends in the Klinefelter Syndrome Treatment Market
With changing trends evidenced by medical advancements, personalized healthcare and increasing emphasis on addressing special needs associated with Klinefelter Syndrome (KS), the treatment market has been affected. One of these trends is the increased awareness and early discovery of KS. Healthcare professionals have become more informed about this condition following a raising level of awareness among them. Over time, there has therefore been an increase in the number of patients suspected to have KS. This is because it becomes easier to intervene promptly when children are identified as having it at school age reducing some health problems related to it. Klinefelter’s syndrome is increasingly becoming a field for personalized medicine especially within its treatment market. An example where this trend emerged has been highlighted through recognizing that symptoms and risks may vary between individuals having KS; therefore, therapeutic approaches should be more specific towards addressing different aspects of this disorder. Thus, hormonal therapy would involve the use HRT for optimizing testosterone, treating infertility while other medications could address osteoporosis or heart issues for example. This approach aims at improving outcomes and quality life among persons living with KS.
Assisted reproductive technologies (ART) have grown in importance concerning Klinefelter Syndrome treatments particularly those focusing on infertility arising from it. However, this case study involving women suffering from KS who have undergone fertilization via IVF-ICSI procedure distinguishes itself from others due to various reasons. It supports recent breakthroughs on how human beings reproduce thereby giving hope to family planning which poses a major challenge among those who suffer from Klinefelter’s syndrome.
The psychosocial support services coupled with mental health considerations are increasingly taking the central parts in the treatment of Klinefelter Syndrome (KS). There appears to be a growing consensus among healthcare providers that counselling should form part of the overall management approach used by doctors in caring for people with Klinefelter Syndrome. All these components being dealt with holistically would help patients facing both physical and psychological problems associated with KS as well as their immediate families thereby promoting comprehensive care models that focus on patients experiencing this condition.
Treatment trends in Klinefelter’s syndrome have been affected by genetic research and molecular mechanisms insights. Consequently, as knowledge about its hereditary basis unfolds, it could become possible to develop targeted therapies addressing specific genes that cause KS. This is also an example of a broader trend in terms of precision medicine which could lead to more focused and efficient treatments designed for a patient’s unique genetic profile.




Leave a Comment