Strengthening Regulatory Frameworks
The regulatory landscape in Japan is evolving to better accommodate the recombinant proteins market. Recent reforms aim to streamline the approval process for biopharmaceuticals, thereby encouraging innovation and expediting the introduction of new therapies. The Pharmaceuticals and Medical Devices Agency (PMDA) has implemented guidelines that facilitate faster review times for recombinant protein products, which is likely to enhance market access for developers. As a result, the strengthening of regulatory frameworks is expected to foster a more conducive environment for the recombinant proteins market, promoting growth and ensuring that patients have timely access to novel therapies.
Growing Focus on Personalized Medicine
The shift towards personalized medicine is significantly influencing the recombinant proteins market in Japan. As healthcare moves away from a one-size-fits-all approach, there is an increasing demand for tailored therapies that cater to individual patient needs. This trend is particularly relevant in oncology and rare diseases, where recombinant proteins can be engineered to target specific genetic profiles. The Japanese government has recognized the importance of personalized medicine and is investing in initiatives to support its development. By 2025, the market for personalized medicine is projected to reach $10 billion, with recombinant proteins playing a pivotal role. Thus, the growing focus on personalized medicine is a key driver for the recombinant proteins market, shaping the future of therapeutic development.
Increasing Investment in Biopharmaceuticals
The recombinant proteins market in Japan is experiencing a surge in investment, particularly in the biopharmaceutical sector. This trend is driven by the growing recognition of the therapeutic potential of recombinant proteins in treating various diseases. In 2025, the biopharmaceutical market in Japan is projected to reach approximately $50 billion, with recombinant proteins accounting for a substantial share. The Japanese government has been actively promoting research and development initiatives, providing funding and incentives to biotechnology firms. This financial support is likely to enhance the capabilities of local companies, fostering innovation and accelerating the development of new recombinant protein therapies. As a result, the increasing investment in biopharmaceuticals is a key driver for the recombinant proteins market, positioning Japan as a leader in this field.
Aging Population and Rising Healthcare Needs
Japan's demographic landscape is characterized by a rapidly aging population, which is expected to significantly impact the recombinant proteins market. By 2025, it is estimated that over 30% of the population will be aged 65 and older, leading to an increased prevalence of chronic diseases. This demographic shift necessitates advanced therapeutic solutions, including recombinant proteins, to address the healthcare needs of the elderly. The demand for innovative treatments is likely to rise, as healthcare providers seek effective options for managing age-related conditions. Consequently, the aging population is a crucial driver for the recombinant proteins market, as it compels the healthcare system to adopt more sophisticated therapeutic approaches.
Technological Advancements in Protein Engineering
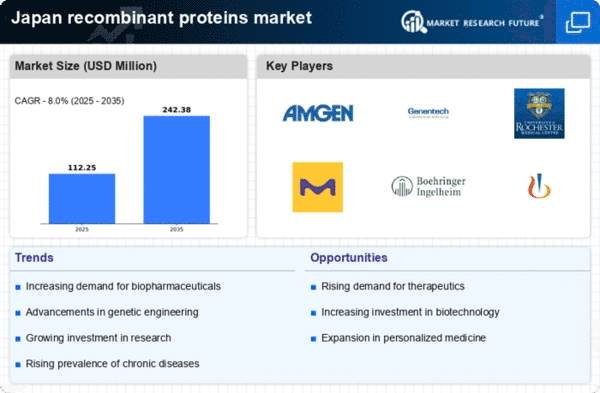
The recombinant proteins market is being propelled by significant technological advancements in protein engineering. Innovations such as CRISPR gene editing and high-throughput screening are enhancing the ability to design and produce recombinant proteins with improved efficacy and safety profiles. In Japan, research institutions and biotechnology companies are increasingly adopting these technologies, leading to the development of novel therapeutic proteins. The market is expected to grow at a CAGR of around 8% from 2025 to 2030, driven by these advancements. As a result, the integration of cutting-edge technologies in protein engineering is a vital driver for the recombinant proteins market, facilitating the creation of next-generation therapeutics.