Aging Population
The demographic shift towards an aging population is significantly influencing the Intravenous Solution Market. Older adults are more susceptible to various health issues, including dehydration and chronic illnesses, which often require intravenous therapy. It is estimated that by 2030, the number of individuals aged 60 and above will surpass 1 billion, leading to a heightened demand for healthcare services, including intravenous solutions. This demographic trend suggests that healthcare systems will increasingly incorporate IV therapies as a standard treatment modality. Consequently, the Intravenous Solution Market is poised for growth as providers adapt to the needs of an aging population, ensuring that effective and timely intravenous solutions are readily available.
Regulatory Support for IV Solutions
Regulatory frameworks supporting the development and approval of intravenous solutions are playing a crucial role in the Intravenous Solution Market. Governments and health authorities are increasingly recognizing the need for safe and effective IV therapies, leading to streamlined approval processes for new products. This regulatory support encourages innovation and investment in the development of advanced intravenous solutions. As a result, the market is witnessing a proliferation of new products designed to meet diverse patient needs. The favorable regulatory environment is expected to bolster the growth of the Intravenous Solution Market, as manufacturers are incentivized to introduce innovative solutions that enhance patient care and treatment outcomes.
Rising Demand for Nutritional Support
The increasing recognition of the importance of nutritional support in patient care is driving growth in the Intravenous Solution Market. Intravenous nutrition, particularly parenteral nutrition, is essential for patients who cannot consume food orally due to various medical conditions. The market for parenteral nutrition solutions is expanding, with projections indicating a growth rate of around 8% annually. This trend is fueled by the rising prevalence of malnutrition in hospitalized patients and the need for tailored nutritional interventions. As healthcare providers prioritize nutritional support as part of comprehensive patient care, the Intravenous Solution Market is likely to see a corresponding increase in the demand for specialized IV nutritional solutions.
Advancements in IV Therapy Technologies
Technological innovations in intravenous therapy are reshaping the Intravenous Solution Market. Developments such as smart infusion pumps, automated IV systems, and enhanced safety features are improving the efficiency and safety of IV administration. These advancements not only reduce the risk of medication errors but also enhance patient comfort and satisfaction. The market for smart infusion devices is projected to grow significantly, with estimates suggesting a compound annual growth rate of over 10% in the coming years. As healthcare facilities adopt these technologies, the Intravenous Solution Market is likely to experience a surge in demand for advanced IV solutions that integrate seamlessly with modern medical practices.
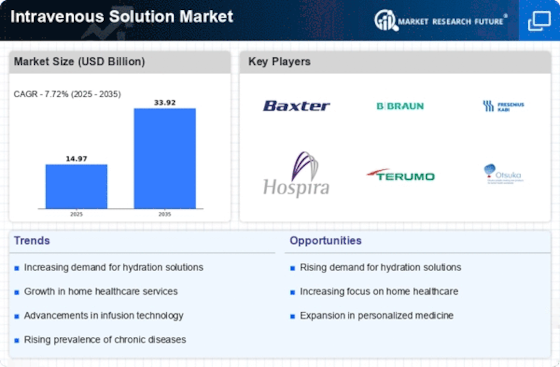
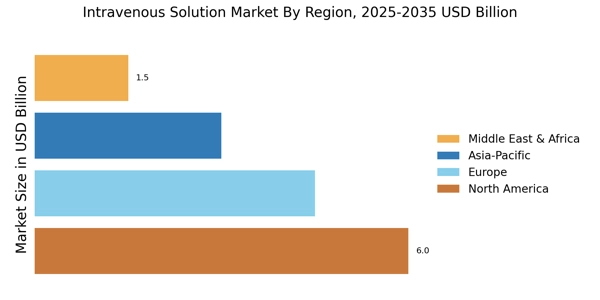
Increasing Prevalence of Chronic Diseases
The rising incidence of chronic diseases such as diabetes, cancer, and cardiovascular disorders is a primary driver of the Intravenous Solution Market. As these conditions often require long-term treatment, the demand for intravenous solutions is expected to grow. According to recent data, chronic diseases account for approximately 60% of all deaths worldwide, necessitating effective management strategies. This trend indicates a sustained need for intravenous therapies, which are crucial for delivering medications, fluids, and nutrients directly into the bloodstream. The Intravenous Solution Market is likely to expand as healthcare providers increasingly rely on IV solutions to manage complex patient needs, thereby enhancing patient outcomes and improving quality of life.