Rising Demand for Biologics
The increasing demand for biologics in Italy is a crucial driver for the healthcare cold-chain-monitoring market. Biologics, which include vaccines, blood products, and gene therapies, require stringent temperature control during storage and transportation. As the Italian healthcare system expands its focus on personalized medicine and advanced therapies, the need for effective cold-chain solutions becomes paramount. The market for biologics in Italy is projected to grow at a CAGR of approximately 8% over the next few years, further emphasizing the necessity for reliable cold-chain monitoring systems. This growth is likely to stimulate investments in advanced monitoring technologies, ensuring that products remain within specified temperature ranges, thereby safeguarding patient safety and product efficacy.
Increased Focus on Patient Safety
Patient safety remains a top priority within the Italian healthcare system, driving the demand for robust cold-chain monitoring solutions. The healthcare cold-chain-monitoring market is significantly influenced by the need to prevent temperature excursions that could compromise the integrity of temperature-sensitive products. Regulatory bodies in Italy are increasingly emphasizing the importance of maintaining proper storage conditions for pharmaceuticals and biologics. As a result, healthcare providers are investing in advanced monitoring systems that provide real-time data and alerts, ensuring compliance with safety standards. This heightened focus on patient safety is expected to propel the market forward, as stakeholders recognize the critical role of cold-chain monitoring in protecting public health.
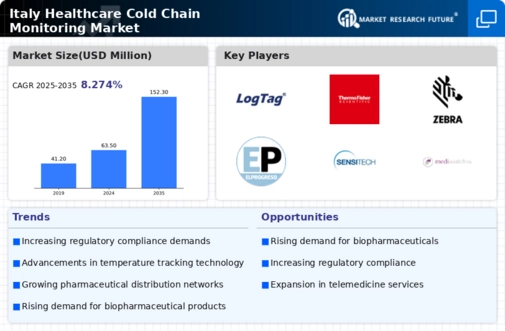
Regulatory Pressure for Compliance
Regulatory pressure is a significant factor influencing the healthcare cold-chain-monitoring market in Italy. Authorities are implementing stringent guidelines to ensure the safe handling and transportation of temperature-sensitive products. Compliance with these regulations is essential for healthcare providers and logistics companies, as non-compliance can result in severe penalties and product recalls. The healthcare cold-chain-monitoring market is responding to this pressure by developing advanced monitoring solutions that facilitate compliance with regulatory standards. As regulations continue to evolve, the market is expected to see increased demand for innovative technologies that provide accurate temperature monitoring and reporting capabilities, ensuring that stakeholders can meet the necessary compliance requirements.
Expansion of E-commerce in Pharmaceuticals
The rapid expansion of e-commerce in the pharmaceutical sector is a significant driver for the healthcare cold-chain-monitoring market in Italy. As more consumers turn to online platforms for purchasing medications and health products, the demand for effective cold-chain solutions has surged. E-commerce companies are increasingly required to ensure that temperature-sensitive products are delivered safely and efficiently. This trend is likely to lead to a rise in investments in cold-chain monitoring technologies, as businesses seek to meet consumer expectations and regulatory requirements. The e-commerce pharmaceutical market in Italy is anticipated to grow by approximately 15% annually, further underscoring the need for reliable cold-chain logistics to support this burgeoning sector.
Technological Integration in Supply Chains
The integration of advanced technologies into supply chains is transforming the healthcare cold-chain-monitoring market in Italy. Innovations such as IoT devices, blockchain, and AI are enhancing the efficiency and reliability of cold-chain logistics. These technologies enable real-time tracking and monitoring of temperature-sensitive products, providing stakeholders with valuable insights into supply chain performance. The adoption of IoT solutions, for instance, is projected to increase by over 30% in the next few years, reflecting a growing trend towards digitalization in healthcare logistics. This technological integration not only improves operational efficiency but also enhances transparency and accountability, which are essential for maintaining product quality and compliance with regulatory standards.

















Leave a Comment