Rising Demand for Biologics
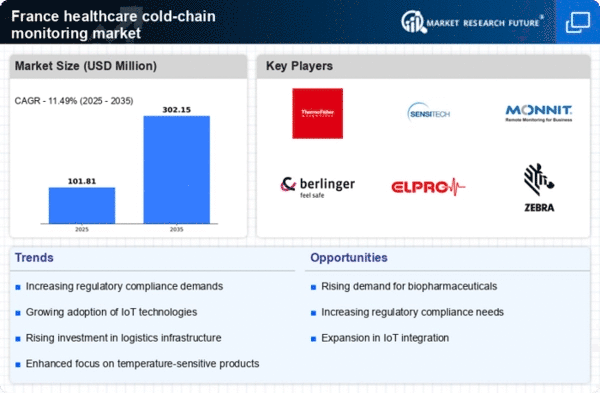
The increasing demand for biologics in France is a pivotal driver for the healthcare cold-chain-monitoring market. Biologics, which include vaccines, blood products, and gene therapies, require stringent temperature control during storage and transportation. As the French healthcare system emphasizes personalized medicine and advanced therapies, the need for effective cold-chain solutions becomes paramount. The market for biologics is projected to grow at a CAGR of approximately 8% over the next few years, further intensifying the demand for reliable cold-chain monitoring systems. This growth necessitates the implementation of sophisticated monitoring technologies to ensure product integrity and compliance with safety standards, thereby propelling the healthcare cold-chain-monitoring market forward.
Increased Focus on Patient Safety
The heightened focus on patient safety in France serves as a crucial driver for the healthcare cold-chain-monitoring market. As healthcare providers prioritize the quality and safety of medications and vaccines, the need for effective cold-chain management becomes increasingly evident. Regulatory bodies are enforcing stricter guidelines regarding the storage and transportation of temperature-sensitive products. This regulatory landscape compels healthcare organizations to adopt advanced monitoring solutions to ensure compliance and safeguard patient health. The investment in cold-chain monitoring technologies is likely to increase as healthcare facilities aim to minimize risks associated with product spoilage and ensure optimal therapeutic outcomes, thus propelling the healthcare cold-chain-monitoring market.
Growing Awareness of Environmental Impact
The increasing awareness of environmental sustainability among consumers and healthcare providers in France is influencing the healthcare cold-chain-monitoring market. Stakeholders are recognizing the importance of reducing carbon footprints associated with cold-chain logistics. This awareness is prompting the adoption of eco-friendly packaging and energy-efficient refrigeration systems. As a result, companies are investing in sustainable cold-chain solutions that not only comply with regulatory standards but also align with consumer expectations for environmentally responsible practices. The shift towards sustainability is likely to reshape the market landscape, encouraging innovation in cold-chain technologies and driving growth in the healthcare cold-chain-monitoring market.
Expansion of E-commerce in Pharmaceuticals
The rapid expansion of e-commerce in the pharmaceutical sector in France significantly influences the healthcare cold-chain-monitoring market. With more consumers opting for online purchases of medications and health products, the need for efficient cold-chain logistics has surged. E-commerce platforms require robust cold-chain solutions to maintain the efficacy of temperature-sensitive products during transit. The French e-pharmacy market is expected to reach €1.5 billion by 2026, indicating a substantial opportunity for cold-chain monitoring systems. This trend compels stakeholders to invest in advanced tracking and monitoring technologies to ensure compliance with regulatory requirements and to enhance customer satisfaction, thereby driving growth in the healthcare cold-chain-monitoring market.
Technological Integration in Supply Chains
The integration of advanced technologies into supply chains is transforming the healthcare cold-chain-monitoring market in France. Innovations such as IoT devices, blockchain, and AI are enhancing the efficiency and reliability of cold-chain logistics. These technologies enable real-time monitoring of temperature and humidity levels, ensuring that products remain within specified parameters throughout the supply chain. The adoption of such technologies is expected to grow, with the market for IoT in healthcare projected to reach €30 billion by 2025. This technological evolution not only improves operational efficiency but also enhances traceability and accountability, thereby driving the demand for sophisticated cold-chain monitoring solutions in the healthcare sector.




















Leave a Comment