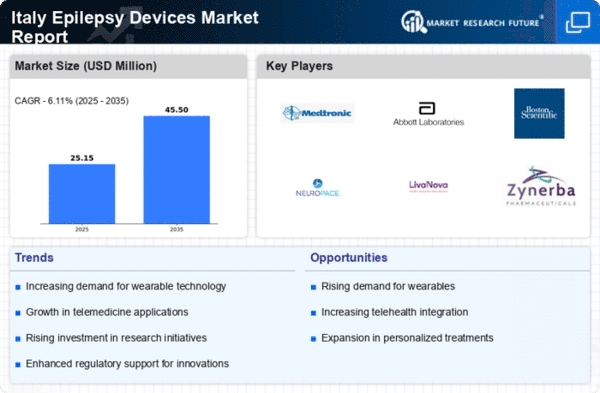
Rising Incidence of Epilepsy
The increasing prevalence of epilepsy in Italy is a crucial driver for the market. Recent estimates suggest that approximately 600,000 individuals in Italy are affected by epilepsy, which translates to about 1% of the population. This rising incidence necessitates the development and adoption of advanced epilepsy devices, as healthcare providers seek effective solutions to manage this condition. The growing patient population is likely to stimulate demand for innovative devices, including seizure detection systems and neurostimulation devices. As awareness of epilepsy continues to grow, the market is expected to expand, with more patients seeking reliable and efficient management options. Consequently, the epilepsy devices market is poised for growth, driven by the urgent need to address the healthcare challenges posed by this neurological disorder.
Increased Awareness and Education
Raising awareness about epilepsy and its management is a vital driver for the epilepsy devices market. In Italy, various organizations and advocacy groups are actively working to educate the public and healthcare professionals about epilepsy. This increased awareness leads to a greater understanding of the condition, reducing stigma and encouraging individuals to seek treatment. As more patients become informed about available devices, the demand for effective epilepsy management solutions is expected to rise. Furthermore, educational initiatives are likely to promote the adoption of advanced devices, as patients and caregivers recognize their potential benefits. The epilepsy devices market will benefit from this heightened awareness, as it fosters a more informed patient population that actively engages in their healthcare decisions.
Supportive Regulatory Environment
The regulatory landscape in Italy is becoming increasingly supportive of the market. Recent initiatives by the Italian government aim to streamline the approval process for medical devices, facilitating quicker access to innovative solutions for patients. This supportive environment encourages manufacturers to invest in research and development, leading to the introduction of new and improved epilepsy devices. Additionally, the government has implemented policies to promote the use of advanced medical technologies in healthcare settings, further driving market growth. As regulatory barriers diminish, the epilepsy devices market is likely to experience accelerated expansion, with more products entering the market to meet the needs of patients and healthcare providers alike.
Advancements in Medical Technology
Technological innovation plays a pivotal role in shaping the epilepsy devices market. The introduction of cutting-edge technologies, such as wearable devices and mobile health applications, has transformed the landscape of epilepsy management in Italy. These advancements enable real-time monitoring of seizures and provide valuable data for healthcare professionals. For instance, devices equipped with artificial intelligence algorithms can analyze seizure patterns and predict potential episodes, enhancing patient safety. The market for these advanced devices is projected to grow significantly, with estimates indicating a compound annual growth rate (CAGR) of around 8% over the next five years. As technology continues to evolve, the epilepsy devices market is likely to witness an influx of innovative solutions that improve patient outcomes and streamline healthcare processes.
Growing Investment in Healthcare Infrastructure
Investment in healthcare infrastructure in Italy is a significant driver for the epilepsy devices market. The government has committed to enhancing healthcare facilities and services, which includes the integration of advanced medical technologies. This investment is expected to improve access to epilepsy devices, particularly in underserved areas. As healthcare facilities upgrade their equipment and adopt new technologies, the demand for innovative epilepsy devices is likely to increase. Furthermore, collaborations between public and private sectors are fostering the development of new solutions tailored to the needs of patients. The epilepsy devices market is poised to benefit from this growing investment, as it aligns with the broader goal of improving healthcare outcomes for individuals living with epilepsy.