Rising Incidence of HPV-Related Cancers
The rising incidence of HPV-related cancers is a significant driver for the HPV CMV Therapeutic Market. HPV is known to be a leading cause of various cancers, including cervical, anal, and oropharyngeal cancers. Recent statistics indicate that HPV-related cancers account for a substantial proportion of cancer cases worldwide, prompting urgent attention from healthcare systems. This alarming trend has led to increased screening and treatment efforts, as well as a growing demand for effective therapeutic interventions. The market is likely to see a surge in the development of targeted therapies aimed at treating HPV-related malignancies, reflecting the urgent need for effective solutions. Consequently, the HPV CMV Therapeutic Market is positioned for growth as stakeholders respond to the rising burden of HPV-related cancers.
Growing Awareness and Education Initiatives
The increasing awareness and education initiatives surrounding HPV and CMV infections are crucial drivers for the HPV CMV Therapeutic Market. Public health campaigns aimed at educating individuals about the risks and prevention of these infections have gained momentum. Organizations and healthcare providers are actively promoting vaccination and screening, which has led to a heightened understanding of the importance of early detection and treatment. This awareness is reflected in the rising vaccination rates for HPV, which have shown a positive trend in recent years. Consequently, as more individuals become informed about their health and the available therapeutic options, the demand for treatments within the HPV CMV Therapeutic Market is expected to rise. This trend underscores the importance of continued education and outreach efforts in driving market growth.
Regulatory Support and Funding for Research
Regulatory support and funding for research initiatives are vital components driving the HPV CMV Therapeutic Market. Government agencies and health organizations are increasingly recognizing the need for effective treatments for HPV and CMV infections, leading to enhanced funding opportunities for research and development. Initiatives aimed at accelerating the approval process for new therapies are also being implemented, which could potentially shorten the time frame for bringing innovative treatments to market. This supportive regulatory environment encourages pharmaceutical companies to invest in research and development, fostering innovation within the HPV CMV Therapeutic Market. As funding increases, researchers are likely to explore novel therapeutic approaches, further expanding the range of available treatment options and enhancing patient care.
Increasing Prevalence of HPV and CMV Infections
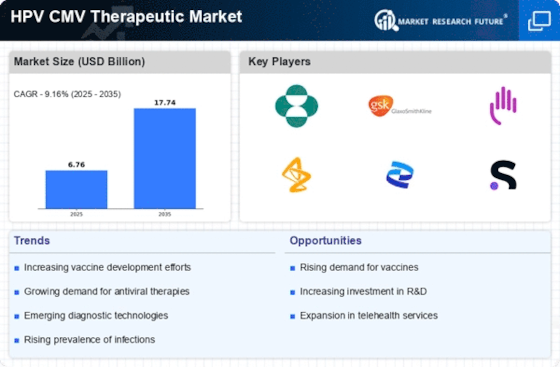
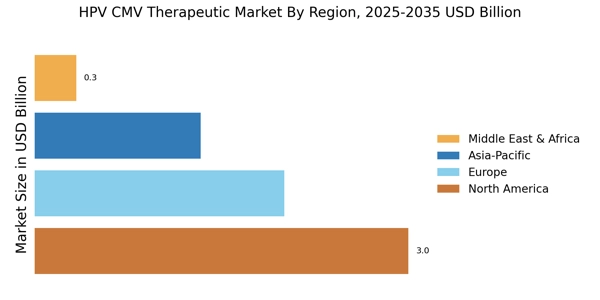
The rising incidence of HPV and CMV infections is a pivotal driver for the HPV CMV Therapeutic Market. Recent estimates indicate that HPV affects approximately 79 million individuals in the United States alone, with around 14 million new infections occurring each year. Similarly, CMV is reported to affect 1 in 150 newborns, leading to significant health complications. This increasing prevalence necessitates the development and availability of effective therapeutic options, thereby propelling market growth. As awareness of these infections grows, healthcare providers are more likely to recommend screening and treatment, further stimulating demand within the HPV CMV Therapeutic Market. The need for innovative therapies to address these infections is becoming increasingly urgent, suggesting a robust market potential for pharmaceutical companies and researchers alike.
Technological Advancements in Treatment Modalities
Technological innovations in treatment modalities are significantly influencing the HPV CMV Therapeutic Market. The advent of novel antiviral agents and therapeutic vaccines has transformed the landscape of treatment options available for HPV and CMV infections. For instance, the development of targeted therapies and immunotherapies has shown promise in enhancing treatment efficacy and patient outcomes. Market data indicates that the antiviral drug segment is expected to witness substantial growth, driven by advancements in drug formulation and delivery systems. These innovations not only improve the effectiveness of existing treatments but also pave the way for the introduction of new therapies, thereby expanding the therapeutic arsenal available to healthcare providers. As a result, the HPV CMV Therapeutic Market is likely to experience a surge in investment and research, fostering a competitive environment for pharmaceutical companies.