Rising Healthcare Expenditure
The upward trend in healthcare expenditure is a significant driver for the Hidradenitis Suppurativa Market. As countries allocate more resources to healthcare, there is an increased focus on chronic conditions like HS, which require long-term management and treatment. This financial commitment is likely to enhance access to advanced therapies and improve patient outcomes. Reports indicate that healthcare spending is expected to rise by approximately 5% annually, which could translate into greater investment in dermatological conditions. Consequently, pharmaceutical companies may be incentivized to develop and market new treatments for HS, thereby expanding the Hidradenitis Suppurativa Market. This financial landscape suggests a promising future for both patients and providers as more resources become available for managing this challenging condition.
Advancements in Treatment Modalities
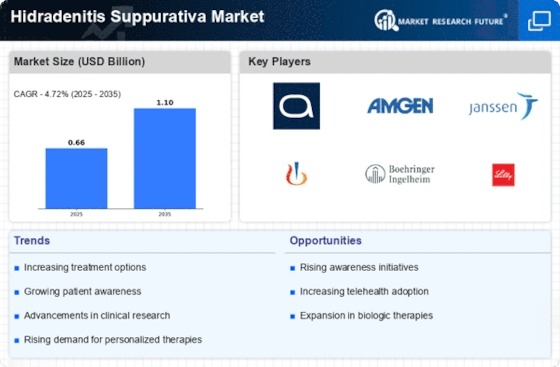
Innovations in treatment modalities are significantly influencing the Hidradenitis Suppurativa Market. The introduction of biologic therapies, such as adalimumab and infliximab, has transformed the management of HS, offering new hope for patients with moderate to severe forms of the disease. These treatments target specific pathways in the inflammatory process, leading to improved outcomes. The market for biologics is projected to grow substantially, with estimates indicating a compound annual growth rate (CAGR) of over 10% in the coming years. Additionally, the development of novel therapies, including small molecules and gene therapies, is on the horizon, which may further enhance treatment options. As these advancements continue to emerge, the Hidradenitis Suppurativa Market is likely to experience a surge in investment and research, fostering a more robust therapeutic landscape.
Integration of Telemedicine in Dermatology
The integration of telemedicine into dermatological practices is emerging as a transformative factor in the Hidradenitis Suppurativa Market. Telehealth services facilitate remote consultations, enabling patients to access specialized care without geographical constraints. This is particularly beneficial for individuals with HS, who may experience discomfort or mobility issues. The convenience of telemedicine is likely to encourage more patients to seek treatment, thereby increasing market demand. Furthermore, the use of digital platforms for monitoring and managing HS can enhance patient engagement and adherence to treatment plans. As telemedicine continues to gain traction, it may reshape the landscape of dermatological care, potentially leading to a more efficient and patient-centered approach in the Hidradenitis Suppurativa Market.
Growing Patient Advocacy and Support Groups
The emergence of patient advocacy and support groups is playing a crucial role in shaping the Hidradenitis Suppurativa Market. These organizations are dedicated to raising awareness about HS, providing education, and advocating for better treatment options. Their efforts have led to increased visibility of the condition, encouraging more individuals to seek diagnosis and treatment. Furthermore, these groups often collaborate with healthcare professionals and pharmaceutical companies to promote research and development initiatives. The presence of such advocacy is likely to drive demand for innovative therapies and improve patient access to care. As the community continues to grow, it may influence policy changes and funding allocations, ultimately benefiting the Hidradenitis Suppurativa Market by fostering a more supportive environment for patients.
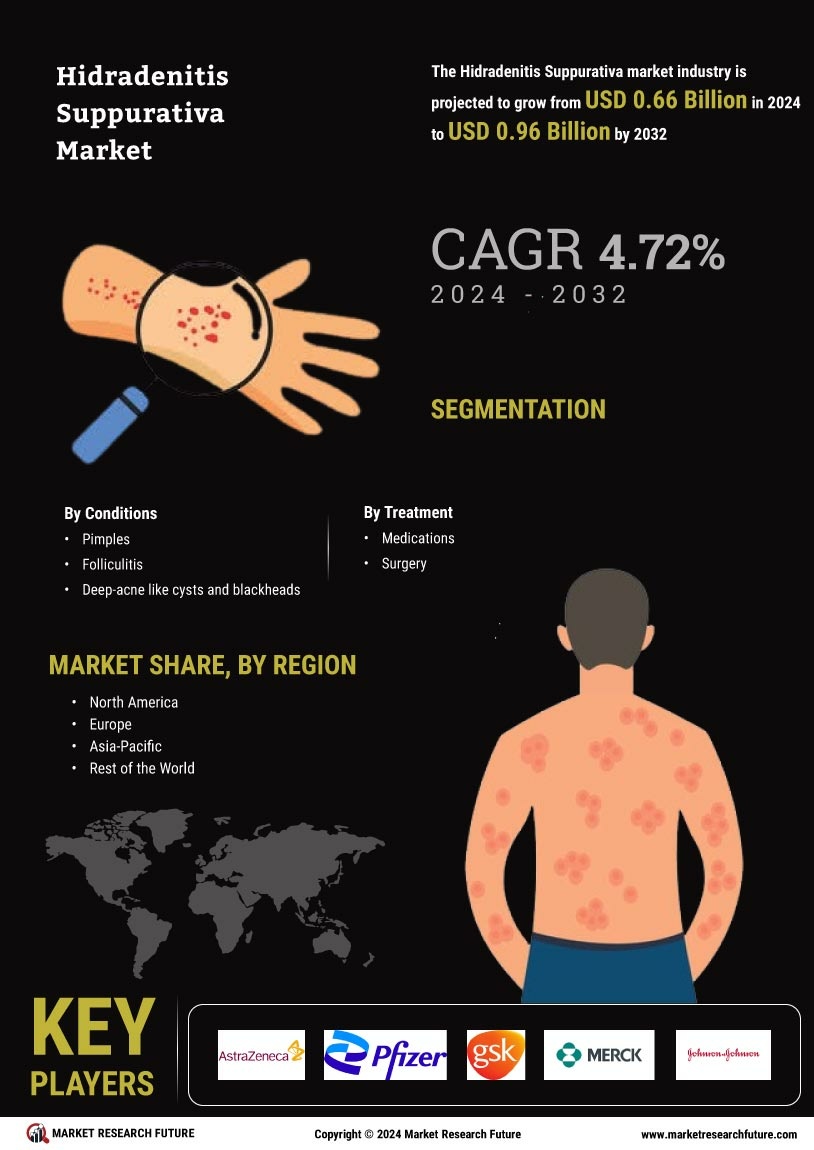
Increasing Prevalence of Hidradenitis Suppurativa
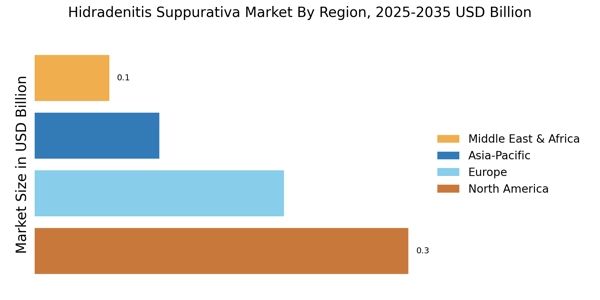
The rising prevalence of Hidradenitis Suppurativa Market (HS) is a notable driver in the Hidradenitis Suppurativa Market. Recent estimates suggest that HS affects approximately 1-4% of the population, with a higher incidence observed in women and individuals aged 20-40. This increasing prevalence is likely to lead to a greater demand for effective treatment options, thereby expanding the market. As awareness grows, more patients are seeking medical attention, which could further contribute to the market's growth. The need for specialized care and management strategies for HS patients is becoming increasingly recognized, indicating a shift in healthcare focus towards this condition. Consequently, the Hidradenitis Suppurativa Market is poised for expansion as healthcare providers adapt to meet the needs of this growing patient population.