Leading market players are investing hugely in research and development in order to expand their product lines, which will help the Healthcare Contract Research Outsourcing (CRO) market grow even more. Market players are also undertaking a variety of strategic activities to spread their global footprint, with important market developments including contractual agreements, new product launches, mergers and acquisitions, higher investments, and collaboration with other organizations. To spread and survive in a more competitive and rising market climate, the Healthcare Contract Research Outsourcing industry must offer cost-effective items.
Manufacturing locally to minimize the operational costs is one of the key business tactics used by the manufacturers in the global Healthcare Contract Research Outsourcing (CRO) industry to benefit clients and increase the market sector. In recent years, the Healthcare Contract Research Outsourcing (CRO) industry has offered some of the most significant advantages to the healthcare industry.
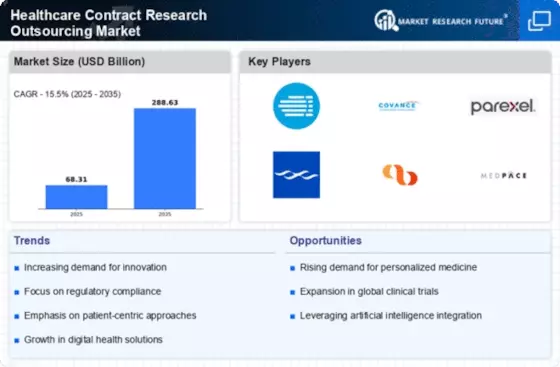
Major participants in the Healthcare Contract Research Outsourcing market, including ICON Plc, Charles River Laboratories, Syneos Health, IQVIA Inc., GVK Biosciences Private Limited, Laboratory Corporation of America Holdings, Parexel International Corporation, Medidata Solutions, Inc., Pharmaron GMBH, and others, are attempting to increase market demand by investing in the research and development operations.
ICON plc is a global contract research organization that offers a broad range of services to pharmaceutical, biotechnology, and medical device companies. ICON is headquartered in Dublin, Ireland, and it operates in numerous countries around the world. This company has invested in technology and data analytics to improve the efficiency and the effectiveness of clinical trials. This includes the use of electronic data capture, ePRO (electronic patient-reported outcomes), and other innovative technologies. In September 2023, ICON and Parexel, two of the world's leading CROs, announced a partnership to develop and deliver integrated clinical trial solutions.
The partnership will put together the two companies' expertise in the clinical trial design, conduct, and analysis to provide clients with a more comprehensive and efficient service.
Catalent, Inc. is a global pharmaceutical services and drug delivery technology company headquartered in Somerset, New Jersey, USA. Catalent provides a wide range of services and solutions to help pharmaceutical, biotechnology, and consumer healthcare companies develop and deliver innovative therapies and products to patients around the world. Catalent invests in developing new drug delivery technologies and enhancing existing ones to address the evolving needs of the pharmaceutical industry.
In March 2023, Catalent, a leading provider of advanced delivery technologies and development solutions for the pharmaceutical, biotechnology, and consumer health industries, acquired Paragon Clinical Development Services, a leading CRO that specializes in late-stage clinical trials. The acquisition expanded Catalent's portfolio of clinical trial services and strengthened its position in the late-stage clinical trial market.

















Leave a Comment