Growing Regulatory Complexity
The contract research organization market is increasingly affected by the growing complexity of regulatory requirements in South Korea. As the healthcare landscape evolves, regulatory bodies are implementing more stringent guidelines to ensure patient safety and data integrity. This complexity necessitates the expertise of contract research organizations, which are well-equipped to navigate the regulatory landscape. In 2025, the demand for regulatory compliance services is expected to rise, with contract research organizations playing a crucial role in assisting clients with the submission of clinical trial applications and adherence to local regulations. This trend indicates that as regulatory frameworks become more intricate, the reliance on contract research organizations will likely increase, further solidifying their position within the market.
Focus on Personalized Medicine
The contract research organization market is significantly influenced by the shift towards personalized medicine in South Korea. As healthcare evolves, there is a growing emphasis on tailoring treatments to individual patient profiles, which necessitates extensive research and data analysis. This trend is expected to drive the market's growth, as contract research organizations play a pivotal role in conducting the necessary studies to support personalized therapies. In 2025, the market for personalized medicine is anticipated to reach $2 billion, with contract research organizations providing essential services such as biomarker identification and patient stratification. This focus on personalized approaches not only enhances treatment efficacy but also aligns with the increasing demand for precision healthcare solutions, thereby reinforcing the importance of contract research organizations in the evolving medical landscape.
Expansion of Biotechnology Sector
The biotechnology sector in South Korea is experiencing rapid expansion, which has a direct impact on the contract research-organization market. With the government investing heavily in biotechnology initiatives, the demand for research services is on the rise. In 2025, the biotechnology market is projected to reach $5 billion, creating a substantial need for contract research organizations to support various stages of drug development. This growth is fueled by advancements in genetic research and the development of novel therapeutics. As biotechnology firms increasingly rely on contract research organizations for clinical trials, regulatory compliance, and data management, the contract research organization market is poised to thrive. This symbiotic relationship between biotechnology and contract research organizations is likely to shape the future of healthcare in South Korea.
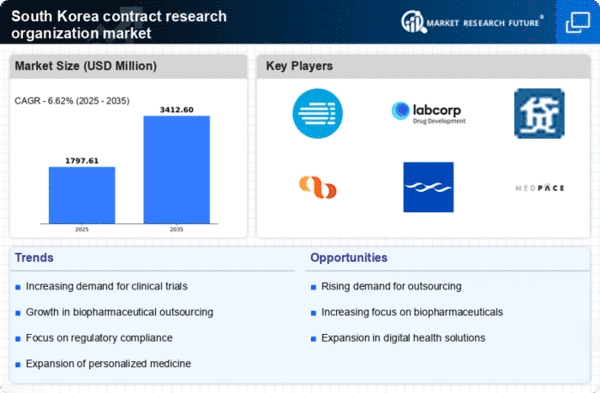
Rising Demand for Clinical Trials
The contract research-organization market in South Korea experiences a notable increase in demand for clinical trials. This surge is driven by the growing need for innovative therapies and the expansion of biopharmaceutical companies. In 2025, the market is projected to reach approximately $1.5 billion, reflecting a compound annual growth rate (CAGR) of around 8%. The increasing prevalence of chronic diseases necessitates extensive research and development, prompting pharmaceutical firms to outsource clinical trial management to contract research organizations. This trend not only enhances efficiency but also allows companies to focus on core competencies while leveraging the expertise of specialized service providers. As a result, the contract research organization market is likely to benefit from this rising demand, positioning itself as a critical player in the healthcare landscape of South Korea.
Increased Investment in Healthcare R&D
Investment in healthcare research and development (R&D) is witnessing a significant uptick in South Korea, which is positively influencing the contract research-organization market. The government and private sector are allocating substantial funds to enhance healthcare innovation, with R&D spending projected to reach $10 billion by 2025. This influx of capital is expected to drive demand for contract research organizations, as pharmaceutical and biotechnology companies seek to outsource their research activities to specialized firms. The emphasis on innovative drug development and advanced therapies necessitates collaboration with contract research organizations, which can provide the expertise and resources required for successful project execution. Consequently, the contract research organization market is likely to benefit from this increased investment, positioning itself as a vital component of the healthcare ecosystem.