Expansion of Clinical Trials
The ongoing expansion of clinical trials is another critical driver influencing the GMP Storage Market. With an increasing number of clinical trials being initiated, the requirement for secure and compliant storage solutions for investigational products is heightened. In 2025, the number of clinical trials is expected to surpass 400,000, creating a substantial demand for GMP-compliant storage facilities. These facilities must ensure the preservation of sensitive materials under stringent conditions, thereby driving investments in advanced storage technologies. As a result, the GMP Storage Market is poised for growth, as stakeholders prioritize compliance and efficiency in their storage operations.
Growing Regulatory Requirements
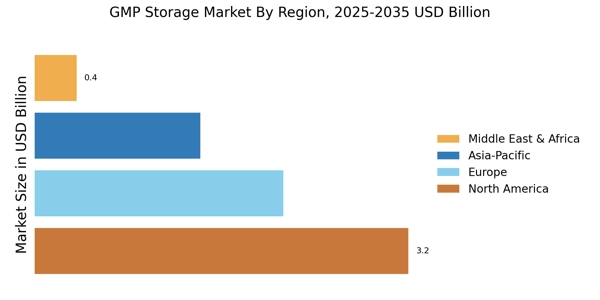
The evolving regulatory landscape is a fundamental driver of the GMP Storage Market. Regulatory agencies are continuously updating guidelines to ensure the safety and efficacy of pharmaceutical products. In 2025, the emphasis on compliance is expected to intensify, with new regulations being introduced that mandate stringent storage conditions. This necessitates the implementation of advanced storage solutions that meet these requirements. As companies strive to adhere to these regulations, the demand for GMP-compliant storage facilities is likely to increase. The GMP Storage Market must remain agile, adapting to these regulatory changes to ensure that storage practices align with the latest standards.
Increased Focus on Quality Assurance
The heightened emphasis on quality assurance within the pharmaceutical sector significantly impacts the GMP Storage Market. As regulatory bodies enforce stricter guidelines, companies are compelled to adopt comprehensive quality management systems. This trend is reflected in the projected growth of the quality assurance market, which is anticipated to reach 10 billion USD by 2025. Consequently, the demand for GMP-compliant storage solutions is likely to rise, as organizations seek to ensure that their products meet the highest quality standards. The GMP Storage Market must adapt to these evolving requirements, fostering innovation in storage technologies to maintain compliance and enhance product quality.
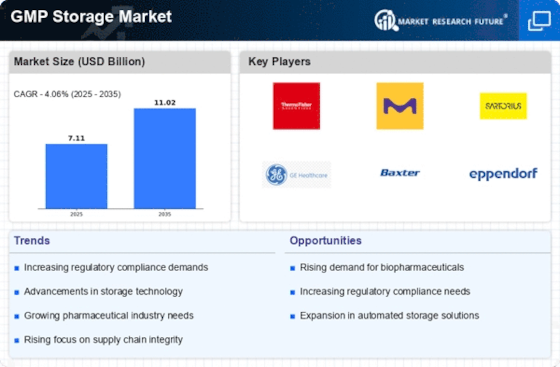
Rising Demand for Biopharmaceuticals
The increasing demand for biopharmaceuticals is a pivotal driver for the GMP Storage Market. As the biopharmaceutical sector expands, the need for compliant storage solutions becomes paramount. In 2025, the biopharmaceutical market is projected to reach approximately 500 billion USD, necessitating robust storage facilities that adhere to Good Manufacturing Practices. This surge in demand compels manufacturers to invest in advanced storage technologies that ensure product integrity and regulatory compliance. Consequently, the GMP Storage Market is likely to experience significant growth as companies seek to optimize their storage capabilities to meet the evolving needs of biopharmaceutical production.
Technological Innovations in Storage Solutions
Technological innovations are transforming the landscape of the GMP Storage Market. The integration of automation, IoT, and advanced monitoring systems is enhancing the efficiency and reliability of storage solutions. In 2025, the market for automated storage systems is expected to grow significantly, driven by the need for real-time monitoring and data integrity. These advancements not only improve operational efficiency but also ensure compliance with stringent regulatory standards. As companies increasingly adopt these technologies, the GMP Storage Market is likely to witness a paradigm shift, with a focus on smart storage solutions that enhance product safety and traceability.