Growing Aging Population
The demographic shift towards an aging population in the GCC region is a significant driver for the catheters active-implantable-cdmo market. Older adults typically experience a higher incidence of chronic conditions that require catheterization, such as cardiovascular diseases and urinary disorders. This demographic trend suggests an increasing need for effective medical devices, including catheters. Projections indicate that the population aged 65 and above in the GCC will double by 2030, creating a substantial market opportunity. Consequently, manufacturers and CDMOs are likely to focus on developing specialized catheters tailored to the needs of this demographic, thereby enhancing market dynamics.
Enhanced Regulatory Frameworks
The catheters active-implantable-cdmo market benefits from enhanced regulatory frameworks established by GCC health authorities. These frameworks aim to ensure the safety and efficacy of medical devices, fostering consumer confidence and encouraging innovation. Regulatory bodies are increasingly streamlining approval processes for new catheter technologies, which may lead to faster market entry for innovative products. This supportive environment is likely to attract investments in research and development, further propelling the market forward. As a result, the catheters active-implantable-cdmo market is expected to witness a steady growth trajectory, driven by a combination of regulatory support and technological advancements.
Rising Healthcare Expenditure in GCC
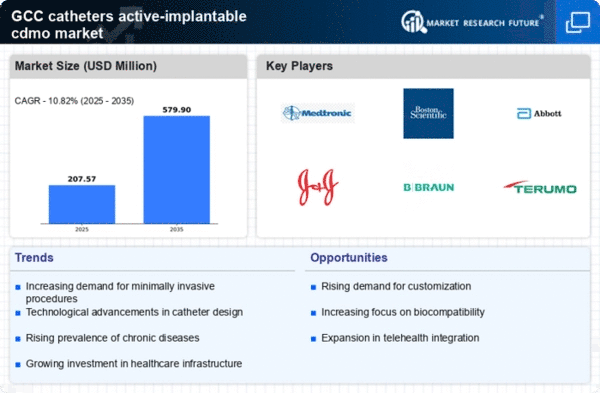
The catheters active-implantable-cdmo market is positively influenced by the rising healthcare expenditure across the GCC countries. Governments are investing heavily in healthcare infrastructure, aiming to enhance service delivery and patient care. This increase in funding facilitates the adoption of advanced medical technologies, including catheters. Reports indicate that healthcare spending in the GCC is expected to reach $100 billion by 2025, which could significantly bolster the market for catheters active-implantable-cdmo. As healthcare facilities upgrade their equipment and services, the demand for high-quality catheters is likely to rise, further driving market growth.
Technological Innovations in Catheter Design
Innovations in catheter design and materials are playing a crucial role in shaping the catheters active-implantable-cdmo market. The introduction of biocompatible materials and advanced manufacturing techniques enhances the performance and safety of catheters. For instance, the development of smart catheters equipped with sensors for real-time monitoring is gaining traction. Such advancements not only improve patient outcomes but also align with the increasing regulatory focus on safety and efficacy. In the GCC, the market is likely to benefit from these technological advancements, with a projected increase in market value reaching $1 billion by 2027. This growth reflects the industry's commitment to innovation and quality.
Increasing Demand for Minimally Invasive Procedures
The catheters active-implantable-cdmo market experiences a notable surge in demand due to the growing preference for minimally invasive procedures. These techniques are associated with reduced recovery times and lower risk of complications, making them increasingly attractive to both patients and healthcare providers. In the GCC region, the healthcare sector is witnessing a shift towards advanced surgical methods, which often necessitate the use of specialized catheters. As a result, the market for catheters active-implantable-cdmo is projected to expand significantly, with estimates suggesting a growth rate of approximately 8% annually. This trend indicates a robust market potential driven by the evolving landscape of surgical practices and patient care.