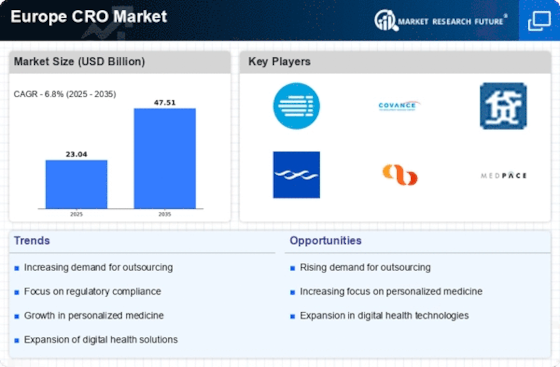
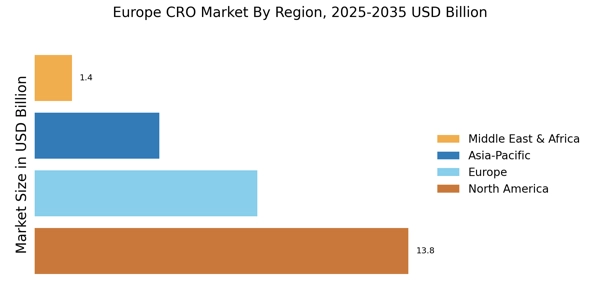
Germany : Strong Growth and Innovation Hub
Key markets include major cities like Berlin, Munich, and Frankfurt, which host numerous pharmaceutical companies and research institutions. The competitive landscape features prominent players such as IQVIA, Covance, and PAREXEL International, all of which have established significant operations in the region. Local market dynamics are characterized by a collaborative environment between academia and industry, fostering innovation in sectors like biotechnology and medical devices.
UK : Innovation and Regulatory Support
Key markets include London, Cambridge, and Manchester, which are hubs for pharmaceutical research and development. The competitive landscape is dominated by major players like IQVIA and PAREXEL International, alongside emerging local firms. The business environment is dynamic, with a focus on collaboration between universities and industry, particularly in sectors like gene therapy and digital health solutions.
France : Strong Regulatory Frameworks
Key markets include Paris, Lyon, and Marseille, which are central to pharmaceutical research and development. The competitive landscape features significant players like PAREXEL International and Charles River Laboratories. The local market dynamics are characterized by a collaborative approach between public and private sectors, particularly in the fields of oncology and neurology, fostering innovation and growth.
Russia : Regulatory Changes and Opportunities
Key markets include Moscow and St. Petersburg, which are central to pharmaceutical research. The competitive landscape is evolving, with both local and international players like Medpace and PPD establishing a presence. The business environment is improving, with a focus on developing local capabilities in sectors such as oncology and infectious diseases, creating new opportunities for growth.
Italy : Focus on Clinical Trials and Research
Key markets include Milan, Rome, and Bologna, which are hubs for pharmaceutical research. The competitive landscape features players like Charles River Laboratories and PAREXEL International. The local market dynamics are characterized by a collaborative environment between research institutions and industry, particularly in sectors like regenerative medicine and rare diseases, fostering innovation and growth.
Spain : Regulatory Support and Growth Potential
Key markets include Madrid and Barcelona, which are central to pharmaceutical research. The competitive landscape features players like IQVIA and Covance, alongside local firms. The business environment is evolving, with a focus on collaboration between public institutions and private companies, particularly in sectors like oncology and infectious diseases, creating new opportunities for growth.
Rest of Europe : Diverse Markets and Unique Challenges
Key markets include smaller nations like Belgium, Switzerland, and the Netherlands, which have emerging pharmaceutical sectors. The competitive landscape is fragmented, with a mix of local and international players. Local market dynamics vary significantly, with some countries focusing on specific therapeutic areas, such as rare diseases or personalized medicine, creating unique challenges and opportunities for CROs.