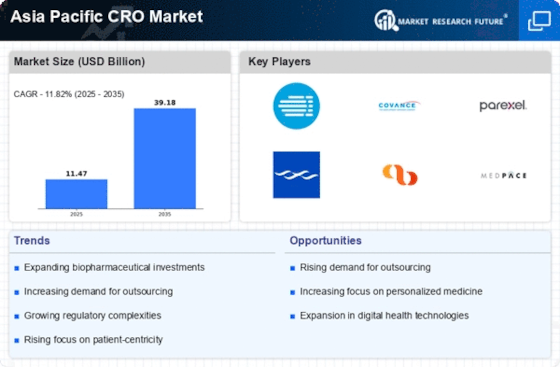
China : Unmatched Growth and Investment Potential
Key markets include Beijing, Shanghai, and Guangzhou, where major players like Wuxi AppTec and IQVIA have established a strong presence. The competitive landscape is characterized by a mix of local and international firms, fostering innovation and collaboration. The business environment is favorable, with streamlined regulatory processes and a growing emphasis on data integrity. The pharmaceutical sector, particularly in drug development and testing, is a significant driver of demand.
India : Rapid Growth in Research Activities
Key cities such as Bengaluru, Hyderabad, and Mumbai are pivotal in the CRO landscape, hosting major players like Syneos Health and PAREXEL. The competitive environment is vibrant, with numerous local firms emerging alongside established international players. The business climate is improving, supported by favorable regulations and a skilled workforce. The biotechnology and pharmaceutical sectors are particularly active, driving demand for clinical research services.
Japan : Strong Focus on Quality and Compliance
Tokyo and Osaka are key markets, with major players like Charles River Laboratories and Medpace leading the charge. The competitive landscape is marked by a blend of local and international firms, fostering a collaborative environment. The business environment is stable, with stringent regulations ensuring high-quality research. The pharmaceutical sector, especially in drug development, is a significant contributor to the CRO market.
South Korea : Strong Government Support and Innovation
Seoul and Busan are central to the CRO market, with significant presence from firms like Novotech and Covance. The competitive landscape features a mix of local and international players, fostering innovation and collaboration. The business environment is favorable, with supportive regulations and a skilled workforce. The pharmaceutical and biotechnology sectors are key drivers of demand for CRO services.
Malaysia : Focus on Regional Collaboration and Growth
Kuala Lumpur and Penang are key markets, with local players and international firms like IQVIA establishing operations. The competitive landscape is evolving, with a mix of established and emerging companies. The business environment is improving, aided by favorable regulations and a growing emphasis on quality standards. The pharmaceutical sector is a significant contributor to the CRO market, driving demand for research services.
Thailand : Strategic Location and Growing Demand
Bangkok and Chiang Mai are key markets, with local firms and international players like Clinipace establishing a presence. The competitive landscape is characterized by a mix of local and foreign companies, fostering collaboration and innovation. The business environment is improving, supported by favorable regulations and a growing emphasis on quality. The pharmaceutical sector is a key driver of demand for CRO services.
Indonesia : Growing Interest and Investment Opportunities
Jakarta and Surabaya are key markets, with emerging local firms and international players beginning to establish a foothold. The competitive landscape is still developing, with opportunities for growth and collaboration. The business environment is improving, aided by regulatory reforms and a focus on quality standards. The pharmaceutical sector is a significant driver of demand for CRO services, particularly in drug development.
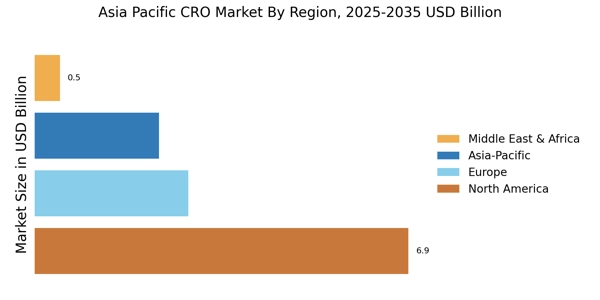
Rest of APAC : Emerging Markets and Growth Potential
Countries like Vietnam, Philippines, and Singapore are key players in this sub-region, with local firms and international companies exploring opportunities. The competitive landscape is varied, with a mix of established and emerging players. The business environment is improving, supported by favorable regulations and a growing emphasis on quality. The pharmaceutical sector is a significant contributor to the CRO market, driving demand for research services.