Leading market players are investing heavily in research and development in order to expand their product lines, which will help the endoscopic retrograde cholangiopancreatography market, grow even more. Market participants are also undertaking a variety of strategic activities to expand their footprint, with important market developments including new product launches, contractual agreements, mergers and acquisitions, higher investments, and collaboration with other organizations. To expand and survive in a more competitive and rising market climate, endoscopic retrograde cholangiopancreatography industry must offer cost-effective items.
Manufacturing locally to minimize operational costs is one of the key business tactics used by manufacturers in the endoscopic retrograde cholangiopancreatography industry to benefit clients and increase the market sector. In recent years, the endoscopic retrograde cholangiopancreatography industry has offered some of the most significant advantages to market.
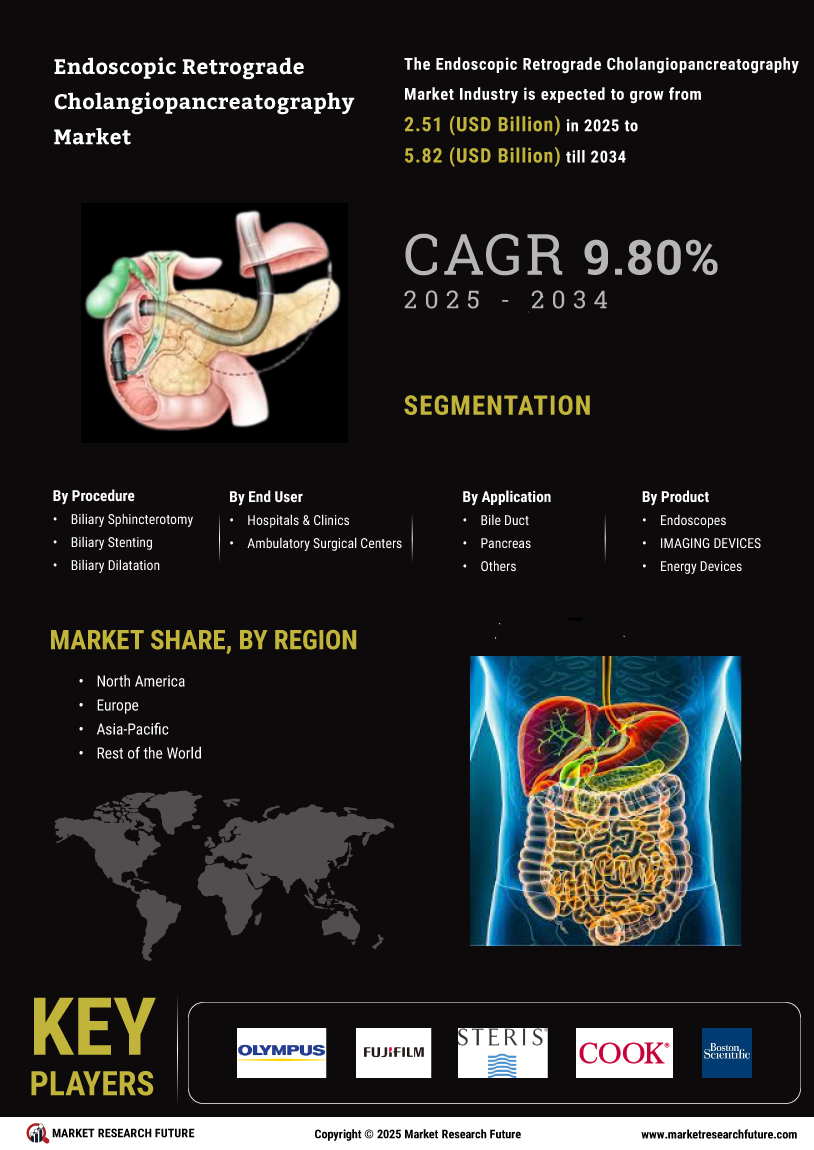
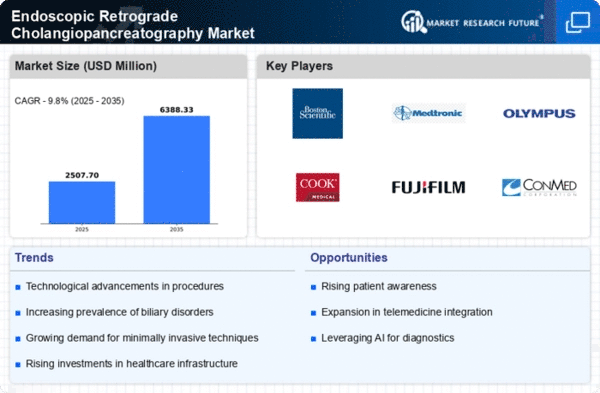
Major players in the endoscopic retrograde cholangiopancreatography market attempting to increase market demand by investing in research and development operations include Olympus Corporation (Japan), Fujifilm Holdings, Corporation (Japan), STERIS PLC (UK), Cook Medical (US), Boston Scientific Corporation (US), CONMED Corporation (US), HOYA Group (Japan), Q3 Medical Devices Limited (Ireland), Ambu Inc. (Denmark), Medtronic Plc (Ireland), and Others.
A corporation called Olympus Corp (Olympus) manufactures precise equipment and tools for optical and digital technologies. The company designs, manufactures, and distributes equipment and devices for the healthcare, information, medical, imaging, and other industrial industries. It offers medical systems like endotherapy devices, endoscopes, and endoscopy supplies to aid medical professionals in treating patients, as well as scientific solutions like laser scanning microscopes, remote visual inspection products, industrial and biological microscope systems, and non-destructive testing systems. In February 2020, the FDA has given Olympus' TJF-Q190V duodenoscope approval.
The product provides an antiseptic and disposable distal endcap made to reduce contamination and improve machine reprocessing.
ConMed Corp (ConMed) is a producer and distributor of surgical tools and instruments for minimally invasive treatments and monitoring. Arthroscopes, bronchoscopy tools, reconstructive systems, endoscopic ligator kits, trocars, balloons for stone removal, graspers, and scissors, suction irrigation tools, thermal tissue fusion systems, electrosurgical generators, pencil sheaths, smoke evacuation pencils, saws, and blades, endo mechanical instrumentation, and surgical visualization tools are some of the company's main products.












Leave a Comment