Market Trends
Key Emerging Trends in the Embolic Protection Devices Market
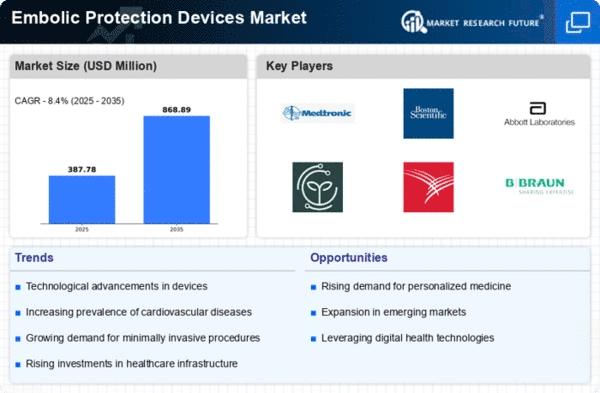
The market trends of embolic protection devices have been undergoing notable transformations, reflecting advancements in medical technology and the growing awareness of cardiovascular diseases. Embolic protection devices play a crucial role in preventing the migration of emboli during vascular procedures, particularly in interventions such as carotid artery stenting and transcatheter aortic valve replacement. A major market factor, when this is considered, is the growing number of cardiovascular diseases in the world. In the face of growing rate of use of embolic protection devices treatment of atherosclerosis among others is on the rise .
The direction of care has significantly been altered in the last several years through the use of minimally invasive procedures with embolic protection devices used as technology implication. Both patients and the health workers can assess the advantages of having a less invasive approach that usually lead to reduced post-operative care times and there are few complications. The trend to decrease invasiveness prompted a rise in demand for embolic protection devices to be deployed in varied cardiovascular procedures. Apart from the fact that the aging population in several developed areas, produced the escalating number of the cases of cardiovascular diseases, which caused a big supply for stent retriever devices.
The strategic relationship between medical device manufacturers and medical facilities is another thing to take into account. Partnership deals and cooperation foster research and development work coordinated with the hopes that the most modern embolic protection devices will meet the needs of the clinical practice. By adding this cooperative method, it's possible to get quicker operations to get new devices approved which, in return, will expedite their entry into the market.
Moreover, patient outcomes and, especially net health system spending make their impact increasingly important in embolic protection device market evolutionary processes. Healthcare providers are seeking solutions that not only demonstrate clinical effectiveness but also contribute to overall cost savings and improved patient outcomes. This shift towards value-based care aligns with the broader trend in the healthcare industry, emphasizing a more patient-centric approach.
















Leave a Comment