Market Analysis
In-depth Analysis of Embolic Protection Devices Market Industry Landscape
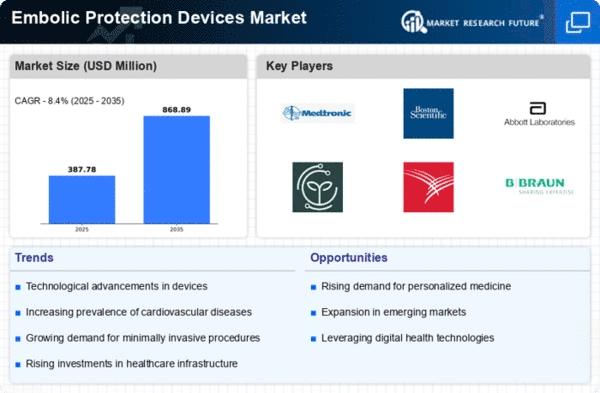
The market dynamics of embolic protection devices (EPDs) reflect a burgeoning healthcare landscape driven by technological advancements and a growing awareness of the importance of cardiovascular health. EPDs are specialized devices designed to prevent the release of embolic debris during interventional procedures, particularly in the field of cardiology. The cardiovascular diseases now spread worldwide, resulting in a demand for quick and less invasive surgery as a market driver for use of EPDs.
One of important factors that drives the market is the decreasing number of people who are diagnosed with coronary artery diseases as well as related disorders. Due to the increase of the elderly and the more sedentary way of life, the frequency of cardiovascular diseases like atherosclerosis has been rising up very highly. This sudden rise in cases is pointing on innovative medical treatment and especially EPDs are being recommended as the key devices to append the formation of thrombus during procedures like angioplasty and stenting.
The advancements in technology have proved to be a variable that has had a limitless impact on the EPD industry. The ever-improving development of new devices with improved facilities have not only revolutionized the usage of embolic protection but also expanded the scope of the market by doing so. Today, innovations like dual-filter systems, bioresorbable devices and progressive materials solve the earlier generation's limitations and enable to epigenetic DNA probe for a broader range of clinical applications.
Alongside with regulatory environment shaping the entire picture of embolic protection devices marketplace, there is another important factor controlling even more their presence on the market. The development of EPDs follows a strict set of rules and regulations and the letusremember.com review process has often been standardized. Not only has this made the devices more secure and efficient but it has also enhanced the sense of confidence among health professionals and patients which subsequently has bolstered the market size. Furthermore, regulatory clearances, and approvals impact favorably the competition among the companies which vies strong market presence through compliance to regulatory norms.
The market dynamics of EPDs are different, and they are affected by such factors as the health system, reimbursement guidelines, and the number of people that have CVDs. Developed regions, with their robust healthcare systems and higher adoption rates of advanced medical technologies, tend to dominate the market share. However, emerging economies are increasingly becoming key growth contributors, driven by rising healthcare awareness, improving infrastructure, and a growing patient pool.
















Leave a Comment