Market Trends and Growth Projections
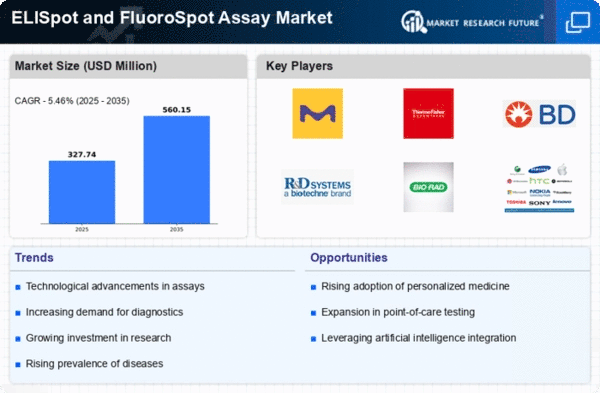
The Global ELISpot and FluoroSpot Assays Market Industry is characterized by dynamic trends and robust growth projections. The market is anticipated to expand significantly, with a valuation of 0.19 USD Billion in 2024 and a forecasted increase to 1.15 USD Billion by 2035. This growth trajectory suggests a compound annual growth rate (CAGR) of 17.99% from 2025 to 2035. Key factors driving this expansion include rising demand for immunological testing, technological advancements, and increased investment in research and development. These trends indicate a promising future for the ELISpot and FluoroSpot assays in various applications.
Expansion of Biopharmaceutical Sector
The expansion of the biopharmaceutical sector significantly impacts the Global ELISpot and FluoroSpot Assays Market Industry, as biopharmaceutical companies increasingly rely on these assays for drug development and clinical trials. The need for accurate immune response assessment in therapeutic interventions drives demand for ELISpot and FluoroSpot assays. With the biopharmaceutical market projected to grow substantially, the assays are expected to play a crucial role in ensuring the efficacy and safety of new therapies. This trend contributes to the market's anticipated growth from 0.19 USD Billion in 2024 to 1.15 USD Billion by 2035.
Rising Demand for Immunological Testing
The Global ELISpot and FluoroSpot Assays Market Industry experiences a notable increase in demand for immunological testing, driven by the growing prevalence of infectious diseases and autoimmune disorders. As healthcare providers seek efficient diagnostic tools, ELISpot and FluoroSpot assays offer precise quantification of antigen-specific T cells, enhancing patient management. In 2024, the market is valued at 0.19 USD Billion, with projections indicating a substantial rise to 1.15 USD Billion by 2035. This growth reflects a compound annual growth rate (CAGR) of 17.99% from 2025 to 2035, underscoring the assays' critical role in modern diagnostics.
Increased Focus on Personalized Medicine
The Global ELISpot and FluoroSpot Assays Market Industry is witnessing a shift towards personalized medicine, where tailored therapeutic approaches require precise immune profiling. As healthcare systems increasingly adopt personalized treatment strategies, the demand for assays that can accurately measure immune responses becomes paramount. ELISpot and FluoroSpot assays provide valuable insights into patient-specific immune profiles, facilitating the development of individualized therapies. This growing focus on personalized medicine is expected to drive the market from 0.19 USD Billion in 2024 to an estimated 1.15 USD Billion by 2035, reflecting a CAGR of 17.99% from 2025 to 2035.
Growing Investment in Research and Development
Investment in research and development (R&D) plays a pivotal role in shaping the Global ELISpot and FluoroSpot Assays Market Industry. Increased funding from government and private sectors fosters innovation in assay technologies, leading to the development of novel applications and improved assay performance. This trend is particularly evident in the field of vaccine development and cancer immunotherapy, where precise immune response monitoring is essential. As R&D efforts intensify, the market is projected to grow from 0.19 USD Billion in 2024 to 1.15 USD Billion by 2035, indicating a robust CAGR of 17.99% from 2025 to 2035.
Technological Advancements in Assay Techniques
Technological advancements significantly influence the Global ELISpot and FluoroSpot Assays Market Industry, as innovations in assay methodologies enhance sensitivity and specificity. The introduction of automated systems and high-throughput screening capabilities allows laboratories to process larger sample volumes efficiently. These advancements not only improve the accuracy of results but also reduce turnaround times, making assays more appealing to clinical and research settings. As a result, the market is poised for growth, with an expected valuation of 1.15 USD Billion by 2035, reflecting the increasing reliance on sophisticated diagnostic tools in immunology.