

- Global Market Outlook
- In-depth analysis of global and regional trends
- Analyze and identify the major players in the market, their market share, key developments, etc.
- To understand the capability of the major players based on products offered, financials, and strategies.
- Identify disrupting products, companies, and trends.
- To identify opportunities in the market.
- Analyze the key challenges in the market.
- Analyze the regional penetration of players, products, and services in the market.
- Comparison of major players’ financial performance.
- Evaluate strategies adopted by major players.
- Recommendations
- Vigorous research methodologies for specific market.
- Knowledge partners across the globe
- Large network of partner consultants.
- Ever-increasing/ Escalating data base with quarterly monitoring of various markets
- Trusted by fortune 500 companies/startups/ universities/organizations
- Large database of 5000+ markets reports.
- Effective and prompt pre- and post-sales support.
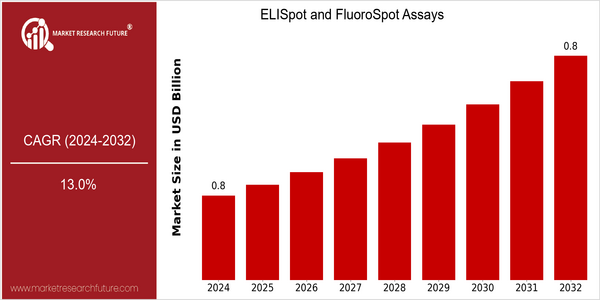
Market Size Snapshot
| Year | Value |
|---|---|
| 2024 | USD 0.8 Billion |
| 2032 | USD 0.8 Billion |
| CAGR (2023-2032) | 13.0 % |
Note – Market size depicts the revenue generated over the financial year
The ELISpot and FluoroSpot Assays market is expected to reach a market size of $800 million by 2024, which is expected to maintain the same value by 2032. This stability in market size, combined with a strong CAGR of 13.0% from 2023 to 2032, is expected to drive the market to the next level. This growth is largely attributed to the increasing focus on the development of a personalized approach to medicine and the need for precise immune response monitoring for the development of vaccines and therapeutics. In addition, the increasing prevalence of infectious diseases and autoimmune disorders is expected to drive the market. The development of multiplexing technology, which allows simultaneous detection of several antigens, is also a factor. In addition, the key players in this market, such as Merck KGaA, BD Biosciences, and Mabtech, are launching new products and entering into strategic alliances to increase their market share. Recent collaborations, for example, that focus on the integration of ELISpot assays with next-generation sequencing, highlight the industry's commitment to advancing diagnostics and improving patient outcomes.
Regional Market Size
Regional Deep Dive
The ELISpot and FluoroSpot Assays Market is experiencing significant growth in the number of regions, owing to the growing demand for advanced diagnostic tools and the rising prevalence of infectious diseases and cancer. In North America, the market is characterized by a strong presence of key players, a strong health care system, and high R & D expenditure. Europe is characterized by a diverse market with a focus on innovation and regulatory compliance. The Asia-Pacific region is expanding rapidly due to increasing healthcare expenditure and a growing trend towards personalized medicine. Middle East and Africa are faced with various challenges, such as access to health care, but they are gradually adopting these assays. Latin America is experiencing an increase in the awareness and use of these assays, which is supported by government initiatives to improve the quality of life.
Europe
- The European Medicines Agency (EMA) has introduced new guidelines for the validation of diagnostic assays, which are influencing the development and commercialization of ELISpot and FluoroSpot technologies across member states.
- Collaborations between academic institutions and biotech companies, such as the partnership between the University of Oxford and Oxford Immunotec, are fostering innovation and expanding the application of these assays in clinical research.
Asia Pacific
- Countries like China and India are witnessing a surge in demand for ELISpot and FluoroSpot assays due to increasing investments in healthcare infrastructure and a growing focus on infectious disease management.
- Local companies, such as Shanghai Kehua Bio-engineering Co., are developing cost-effective assay solutions tailored to regional needs, which is expected to enhance accessibility and adoption in the market.
Latin America
- Government initiatives in Brazil and Mexico aimed at enhancing healthcare access are driving the adoption of advanced diagnostic technologies, including ELISpot and FluoroSpot assays.
- Collaborations between local universities and international biotech firms are fostering innovation and knowledge transfer, which is expected to enhance the capabilities and availability of these assays in the region.
North America
- The U.S. Food and Drug Administration (FDA) has recently streamlined the approval process for diagnostic assays, which is expected to accelerate the introduction of new ELISpot and FluoroSpot products in the market.
- Key players such as Merck KGaA and Bio-Rad Laboratories are investing heavily in R&D to enhance the sensitivity and specificity of their assays, which is likely to drive market growth and innovation.
Middle East And Africa
- The World Health Organization (WHO) has initiated programs to improve disease surveillance in the MEA region, which is likely to increase the demand for ELISpot and FluoroSpot assays as part of diagnostic protocols.
- Emerging biotech firms in South Africa are beginning to develop localized versions of these assays, which could lead to increased market penetration and improved healthcare outcomes in underserved areas.
Did You Know?
“The ELISpot assay is considered one of the most sensitive methods for measuring immune responses, capable of detecting as few as 1-10 cells producing a specific cytokine.” — Journal of Immunological Methods
Segmental Market Size
The market for ELISpot and Flurospot assays is a significant part of the broader field of Immunoassay. It is experiencing a steady growth, driven by the increasing demand for precise immune responses. The increasing prevalence of infectious and autoimmune diseases, as well as the growing importance of individualized medicine, are driving this market. Also, the regulatory support for these assays is conducive to their adoption. The current ELISpot and Flurospot assays are moving from a discovery phase to an implementation phase. In this phase, the notable players, such as Merck and Mabtech, are dominating the market, particularly in the U.S. and Europe. These assays are used primarily in the field of vaccine development, cancer research, and infectious diseases, where they provide crucial insights into the T-cell responses. The macro-developments, such as the growing importance of pandemic preparedness and the need for rapid diagnostics, are conducive to this market. The development of these assays is shaped by the introduction of the newer technologies, such as automation and multiplexing, which are expected to increase their throughput and accuracy, and further solidify their role in the modern immunology research.
Future Outlook
The ELISpot and FluoroSpot assays market is projected to grow at a CAGR of 13.0% between 2024 and 2032. This growth is driven by the rising prevalence of infectious diseases and the growing demand for personalized medicine, which requires advanced immunological assays for precise diagnostics and monitoring of therapy. The market size is projected to reach nearly $800 million by 2032, as a result of the rising adoption of these assays in the clinical and research settings. The growing automation and high-throughput screening capabilities are also expected to increase the efficiency and accuracy of ELISpot and FluoroSpot assays. The rising focus on vaccines and immunotherapy, especially in view of the emerging public health issues, is also expected to boost the demand for these assays. Also, the shift toward point-of-care testing and the incorporation of digital health solutions are expected to transform the market by making these assays more accessible and convenient. The market is evolving, and the key players are trying to remain agile to seize the opportunities and to meet the regulatory challenges and competition.
Covered Aspects:| Report Attribute/Metric | Details |
|---|---|
| Growth Rate | 13% |
ELISpot and FluoroSpot Assay Market Highlights:
Leading companies partner with us for data-driven Insights
Kindly complete the form below to receive a free sample of this Report
Tailored for You
- Dedicated Research on any specifics segment or region.
- Focused Research on specific players in the market.
- Custom Report based only on your requirements.
- Flexibility to add or subtract any chapter in the study.
- Historic data from 2014 and forecasts outlook till 2040.
- Flexibility of providing data/insights in formats (PDF, PPT, Excel).
- Provide cross segmentation in applicable scenario/markets.









