Market Analysis
In-depth Analysis of Dermatophytic Onychomycosis Treatment Market Industry Landscape
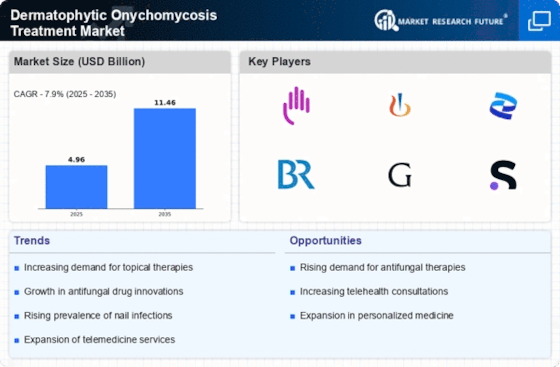
Dermatophytic onychomycosis, a common fungal infection affecting the nails, has spurred a dynamic landscape in the treatment market. The market dynamics of dermatophytic onychomycosis treatment reflect a blend of scientific advancements, consumer demand, regulatory frameworks, and competitive forces. At its core, this market is driven by the rising prevalence of fungal nail infections globally, with millions seeking effective remedies each year.
One of the primary drivers shaping the market dynamics is the growing awareness among both consumers and healthcare professionals about the impact of onychomycosis on quality of life. As people become more conscious of the condition's cosmetic and health implications, the demand for effective treatments rises. This increased awareness has prompted pharmaceutical companies to invest in research and development, leading to the introduction of novel therapies and formulations targeting fungal nail infections.
Moreover, the market dynamics of dermatophytic onychomycosis treatment are heavily influenced by technological advancements and innovations in medical science. The emergence of new antifungal agents, drug delivery systems, and diagnostic tools has expanded the treatment options available to patients. From topical solutions to oral medications and laser therapies, the spectrum of treatments continues to evolve, offering patients a wider array of choices based on their preferences and medical needs.
Furthermore, regulatory factors play a significant role in shaping the landscape of the dermatophytic onychomycosis treatment market. Government agencies such as the FDA (Food and Drug Administration) in the United States and the EMA (European Medicines Agency) in Europe set stringent guidelines for the approval and marketing of pharmaceutical products. Compliance with these regulations not only ensures the safety and efficacy of treatments but also influences market access and commercialization strategies for pharmaceutical companies.
In addition to regulatory considerations, market dynamics are influenced by competitive forces within the pharmaceutical industry. As the prevalence of onychomycosis continues to rise, the market has become increasingly crowded with both established players and new entrants vying for market share. This intense competition drives innovation, pricing strategies, and marketing efforts as companies strive to differentiate their products and gain a competitive edge.
Moreover, market dynamics are shaped by macroeconomic factors such as healthcare expenditure, reimbursement policies, and demographic trends. The willingness of payers to cover the cost of dermatophytic onychomycosis treatment, coupled with shifting demographic patterns such as aging populations, influences the demand for fungal nail infection therapies and the overall growth of the market.
















Leave a Comment