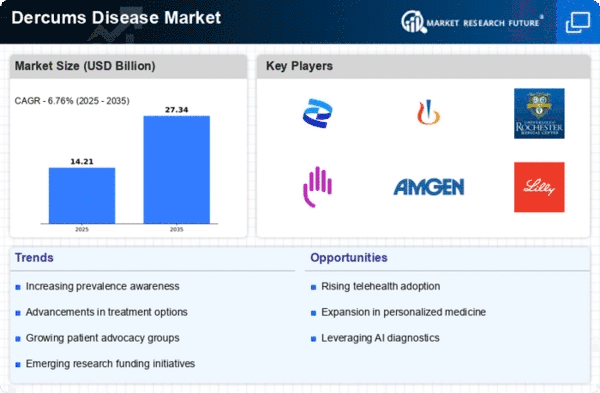
Market Share
Dercums Disease Market Share Analysis
Understanding Target Audience : Identifying the demographics and psychographics of individuals affected by Dercum's Disease is crucial. This includes age, gender, geographical location, income level, and lifestyle factors.
Product Differentiation : Developing unique features or benefits in products or services specifically tailored to address the needs of Dercum's Disease patients. This could involve formulations with specific ingredients proven to alleviate symptoms or innovative delivery methods for medication.
Market Segmentation : Dividing the market into distinct groups based on characteristics such as symptom severity, co-morbidities, or treatment preferences. Tailoring marketing strategies and product offerings to each segment can enhance relevance and appeal.
Collaboration with Healthcare Professionals : Building relationships with healthcare providers specializing in Dercum's Disease can facilitate referrals and endorsement of products or services. This includes physicians, surgeons, physiotherapists, and nutritionists.
Education and Awareness Campaigns : Investing in initiatives to raise awareness about Dercum's Disease among both healthcare professionals and the general public. This could involve organizing seminars, distributing informational materials, and leveraging digital platforms for advocacy.
Patient Support Programs : Establishing support networks and resources for individuals living with Dercum's Disease can foster loyalty and trust. Providing access to counseling services, online forums, and educational materials can enhance the overall patient experience.
Clinical Research and Development : Investing in research initiatives aimed at advancing understanding and treatment options for Dercum's Disease. This includes conducting clinical trials, collaborating with academic institutions, and exploring novel therapeutic approaches.
Price Competitiveness : Balancing the need for profitability with affordability to ensure accessibility for patients. Implementing pricing strategies that reflect the value proposition of products or services while remaining competitive within the market.
Distribution Channels : Optimizing distribution channels to ensure convenient access to products or services for individuals affected by Dercum's Disease. This may involve partnerships with pharmacies, specialty clinics, or online retailers.
Feedback Mechanisms : Establishing channels for gathering feedback from patients, caregivers, and healthcare professionals to continuously improve products or services. This includes soliciting input through surveys, focus groups, and customer support channels.
Regulatory Compliance : Adhering to regulatory requirements and quality standards governing the development, manufacturing, and marketing of products or services for Dercum's Disease. This ensures safety, efficacy, and trust among stakeholders.
Competitive Analysis : Monitoring competitors' activities and market dynamics to identify opportunities and threats. This includes assessing product offerings, pricing strategies, promotional tactics, and market share trends within the Dercum's Disease landscape.

















Leave a Comment