Market Analysis
In-depth Analysis of COVID 19 Sample Collection Kits Market Industry Landscape
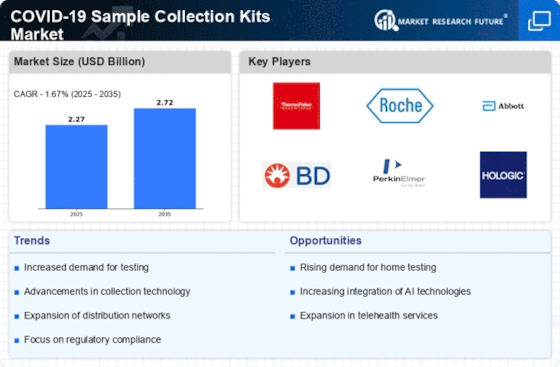
The COVID-19 sample collection kits market has seen dynamic development since the beginning of the pandemic, driven by the worldwide interest for capable and open answers for diagnosing the virus. These kits assume a pivotal part in the collection, conservation, and transportation of natural specimens, working with exact testing for the presence of the SARS-CoV-2 virus. Market elements are firmly affected by the surge in popularity for COVID-19 testing. State run administrations, healthcare associations, and confidential substances overall have motivated testing endeavors to recognize and segregate cases speedily, driving the requirement for dependable sample collection kits. The market incorporates an assortment of sample collection techniques, including nasopharyngeal swabs, oropharyngeal swabs, saliva collection kits, and that is just the beginning. This variety takes special care of various testing inclinations, patient comfort, and healthcare settings, adding to the flexibility and openness of COVID-19 testing. The market is seeing development in self-testing advancements, including at-home collection kits that coordinate with cell phone applications for result announcing. These progresses aim to upgrade client experience, increase availability, and support inevitable testing interest. Market elements are affected by cost contemplations and moderateness. Adjusting the expense viability of sample collection kits with the requirement for far reaching testing is essential for market players to guarantee availability and address monetary difficulties related with the pandemic. The COVID-19 sample collection kits market is ready for proceeded with advancement as the condition and testing needs develop. Continuous progresses in innovation, administrative variations, and key reactions to arising variations of the virus add to the flexibility of sample collection arrangements. The COVID-19 sample collection kits market is supposed to remain dynamic, reflecting continuous endeavors to upgrade testing efficiency, openness, and unwavering quality. Future patterns might remember further headways for self-testing advances, consistent upgrades in component strategy, and the foundation of strong worldwide organizations to guarantee the powerful reaction to general health crises.

















Leave a Comment