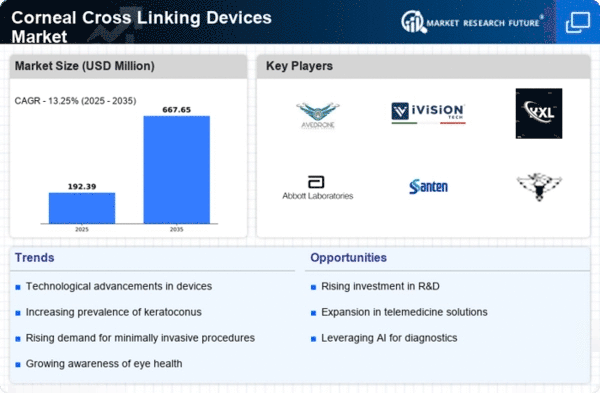
Market Growth Projections
The Global Corneal Cross Linking Devices Market Industry is projected to experience substantial growth in the coming years. With a current valuation of 0.17 USD Billion in 2024, the market is anticipated to expand significantly, reaching an estimated 0.65 USD Billion by 2035. This growth trajectory indicates a compound annual growth rate of 13.02% from 2025 to 2035. Factors contributing to this growth include increasing prevalence of keratoconus, technological advancements, and rising awareness among patients and healthcare providers. The market's expansion reflects a broader trend towards improved eye care and treatment options.
Rising Geriatric Population
The global increase in the geriatric population correlates with a higher prevalence of eye disorders, thereby influencing the Global Corneal Cross Linking Devices Market Industry. Older adults are more susceptible to conditions like keratoconus, necessitating effective treatment options. As the population aged 65 and above continues to grow, the demand for corneal cross linking devices is expected to rise. This demographic shift is likely to drive market growth, with projections indicating a market size of 0.65 USD Billion by 2035. The aging population underscores the need for innovative solutions to address age-related eye conditions.
Growing Awareness and Education
Increased awareness and educational initiatives regarding corneal diseases and their treatments significantly impact the Global Corneal Cross Linking Devices Market Industry. Healthcare organizations and advocacy groups are actively promoting knowledge about keratoconus and the benefits of corneal cross linking. This heightened awareness leads to early diagnosis and treatment, which is crucial for effective management of the condition. As a result, more patients are likely to seek out these procedures, contributing to market growth. The industry is expected to see a valuation of 0.17 USD Billion in 2024, with a potential increase as educational efforts continue to expand.
Regulatory Support and Approvals
Supportive regulatory frameworks and timely approvals for corneal cross linking devices play a crucial role in the Global Corneal Cross Linking Devices Market Industry. Regulatory bodies are increasingly recognizing the importance of these devices in treating keratoconus and other corneal disorders. Streamlined approval processes facilitate quicker market entry for new technologies, encouraging manufacturers to innovate. This regulatory environment fosters competition and enhances patient access to advanced treatment options. As a result, the market is poised for growth, with expectations of reaching 0.65 USD Billion by 2035.
Increasing Prevalence of Keratoconus
The rising incidence of keratoconus globally drives demand for corneal cross linking devices. Keratoconus, a progressive eye disease, affects approximately 1 in 2,000 individuals, leading to significant visual impairment. As awareness of this condition grows, more patients seek treatment options, thereby propelling the Global Corneal Cross Linking Devices Market Industry. In 2024, the market is valued at 0.17 USD Billion, reflecting the urgent need for effective interventions. The increasing prevalence of keratoconus is expected to contribute to a compound annual growth rate of 13.02% from 2025 to 2035, potentially reaching 0.65 USD Billion by 2035.
Technological Advancements in Treatment
Innovations in corneal cross linking technology enhance treatment efficacy and safety, thereby boosting the Global Corneal Cross Linking Devices Market Industry. Newer devices utilize advanced techniques such as accelerated cross linking, which reduces treatment time and improves patient comfort. These advancements not only attract more healthcare providers but also encourage patients to opt for these procedures. As technology continues to evolve, the market is likely to expand, with a projected valuation of 0.65 USD Billion by 2035. The integration of artificial intelligence in treatment planning further indicates a promising future for the industry.