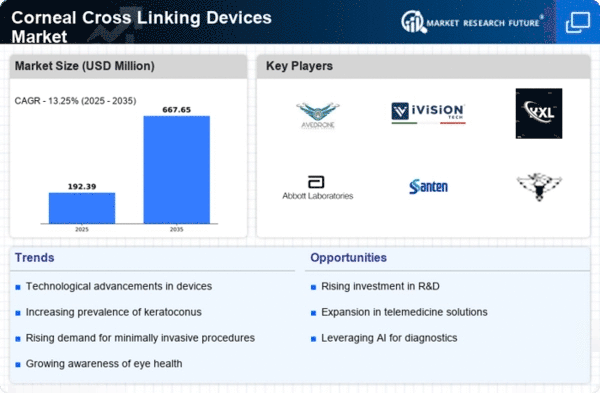
Market Analysis
In-depth Analysis of Corneal Cross Linking Devices Market Industry Landscape
The abundant presence of products in the Corneal Cross-linking (CXL) devices pipeline reflects a robust commitment to research and development, signaling the potential introduction of more effective products into the market. The continuous launch of new and improved products plays a pivotal role in driving market growth. The landscape of Corneal Cross-linking (CXL) devices is witnessing ongoing clinical trials, hinting at the imminent approval of novel solutions in the near future.
An exemplar of this trend is evident in the activities of CXL Ophthalmics, LLC (US), a clinical-stage company specializing in treatments for corneal ectasia. In November 2021, the company unveiled the results of its Phase 2 Trial in transepithelial (Epi-On) cross-linking. The positive outcomes from this trial have propelled the company to prepare for the launch of Phase III studies, a crucial step towards the registration of the EpiSmart cross-linking system in the United States. This initiative underscores the dedication to advancing treatment methodologies in the field.
Similarly, Glaukos Corporation (US), a prominent player in ophthalmic medical technology and pharmaceuticals, made significant strides in February 2021. The company shared positive results from Phase 3 trials for iLink Epi-on, an investigational therapy designed for the treatment of keratoconus. This breakthrough not only contributes to the advancement of treatment options but also showcases the potential for more effective interventions in the near future.
Furthermore, a clinical trial involving the PXL Platinum 330 by Peschke GmbH (Switzerland), titled “Safety and Effectiveness of the PXL Platinum 330 System with Riboflavin Solution for Refractory Corneal Ulcers,” is scheduled to conclude by June 2023. This trial, initiated in March 2022, holds promise for expanding the array of treatment options available for refractory corneal ulcers.
The ongoing product innovations and the anticipation of potential launches underscore the significant growth opportunities on the horizon for the Corneal Cross-linking (CXL) devices market. The continual focus on research and development activities, coupled with the positive outcomes from clinical trials, positions the market for sustained growth in the coming years. As novel and more efficient products emerge, they are expected to contribute not only to the expansion of treatment options but also to the overall advancement of the Corneal Cross-linking (CXL) devices market.

















Leave a Comment