Market Growth Projections
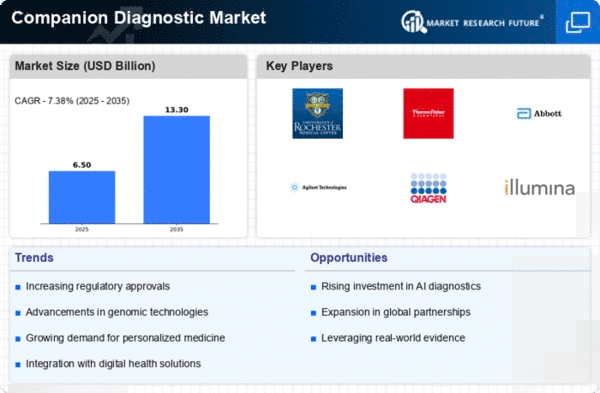
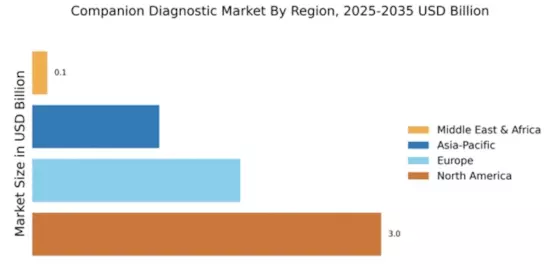
The Global Companion Diagnostics Market Industry is poised for remarkable growth, with projections indicating a market value of 36.8 USD Billion in 2024 and an anticipated increase to 308.0 USD Billion by 2035. This trajectory suggests a compound annual growth rate (CAGR) of 21.32% from 2025 to 2035, reflecting the increasing integration of companion diagnostics into clinical practice. The growth is driven by various factors, including advancements in technology, rising chronic disease prevalence, and regulatory support for personalized medicine. Such projections highlight the dynamic nature of the market and its potential to reshape healthcare delivery.
Increasing Investment in R&D
Investment in research and development is a critical driver of the Global Companion Diagnostics Market Industry. Pharmaceutical companies and biotechnology firms are allocating substantial resources to develop innovative companion diagnostics that can enhance treatment efficacy and patient outcomes. This trend is supported by collaborations between industry stakeholders and academic institutions, fostering an environment conducive to innovation. As a result, the market is poised for significant growth, with projections indicating a market value of 36.8 USD Billion in 2024. The emphasis on R&D is likely to yield breakthroughs that further advance the field of personalized medicine.
Advancements in Genomic Technologies
Technological advancements in genomics are significantly influencing the Global Companion Diagnostics Market Industry. Innovations such as next-generation sequencing and CRISPR gene editing are enabling more precise identification of biomarkers associated with various diseases. These technologies enhance the development of companion diagnostics, allowing for tailored treatment plans that improve patient outcomes. As a result, the market is expected to expand, with projections indicating a growth to 308.0 USD Billion by 2035. The integration of these advanced technologies into clinical practice is likely to drive demand for companion diagnostics, thereby reshaping the landscape of personalized medicine.
Growing Demand for Targeted Therapies
The demand for targeted therapies is a significant factor propelling the Global Companion Diagnostics Market Industry. As healthcare providers increasingly recognize the benefits of personalized treatment approaches, the need for companion diagnostics that can identify the most effective therapies for specific patient populations becomes paramount. This shift towards precision medicine is evident in the rising number of targeted therapies approved by regulatory agencies. The market's growth is underscored by the projected increase in value to 308.0 USD Billion by 2035, reflecting the ongoing transformation in treatment paradigms that prioritize individualized care.
Rising Prevalence of Chronic Diseases
The Global Companion Diagnostics Market Industry is experiencing growth driven by the increasing prevalence of chronic diseases such as cancer, diabetes, and cardiovascular disorders. As these conditions become more widespread, the demand for personalized medicine rises, necessitating the development of companion diagnostics that can identify suitable therapies for individual patients. For instance, the rise in cancer cases has led to a greater need for targeted therapies, which companion diagnostics can facilitate. This trend is reflected in the projected market value of 36.8 USD Billion in 2024, indicating a robust growth trajectory as healthcare systems adapt to these challenges.
Regulatory Support for Personalized Medicine
Regulatory bodies are increasingly supporting the development of personalized medicine, which is a key driver for the Global Companion Diagnostics Market Industry. Initiatives aimed at streamlining the approval process for companion diagnostics encourage innovation and investment in this sector. For example, the FDA has established pathways that expedite the review of companion diagnostics alongside their corresponding therapies. This regulatory environment fosters collaboration between pharmaceutical companies and diagnostic developers, enhancing the market's growth potential. As the industry evolves, the supportive regulatory framework is likely to contribute to the anticipated CAGR of 21.32% from 2025 to 2035.