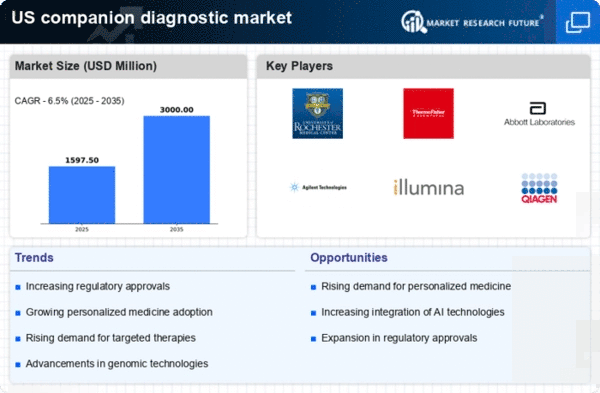
Advancements in Genomic Technologies
Technological innovations in genomic sequencing and molecular diagnostics are significantly influencing the companion diagnostic market. The advent of next-generation sequencing (NGS) has enabled more precise identification of genetic mutations, which is crucial for the development of targeted therapies. In the US, the market for genomic testing is projected to reach approximately $25 billion by 2026, indicating a robust growth trajectory. This growth is likely to enhance the capabilities of the companion diagnostic market, as more sophisticated tests become available, allowing for better patient stratification and treatment personalization.
Rising Prevalence of Chronic Diseases
The increasing incidence of chronic diseases such as cancer, cardiovascular disorders, and diabetes is a primary driver for the companion diagnostic market. As these conditions become more prevalent, the demand for personalized medicine rises, necessitating the use of companion diagnostics to tailor treatments to individual patients. In the US, it is estimated that nearly 1.9 million new cancer cases are diagnosed annually, highlighting the need for effective diagnostic tools. The companion diagnostic market is thus positioned to grow, as healthcare providers seek to improve patient outcomes through targeted therapies that are informed by diagnostic testing.
Growing Demand for Personalized Medicine
The shift towards personalized medicine is reshaping the landscape of the companion diagnostic market. Patients and healthcare providers are increasingly recognizing the value of tailored treatment approaches that consider individual genetic profiles. This trend is supported by a growing body of evidence demonstrating improved treatment efficacy and reduced adverse effects. In the US, the personalized medicine market is expected to exceed $2 trillion by 2025, which could significantly bolster the companion diagnostic market as more healthcare systems adopt these strategies to enhance patient care.
Regulatory Support for Companion Diagnostics
Regulatory bodies in the US are providing enhanced support for the development and approval of companion diagnostics, which is a vital driver for the market. Initiatives aimed at streamlining the regulatory process for these diagnostics are encouraging innovation and expediting time-to-market for new tests. The FDA has established specific pathways for the approval of companion diagnostics, which may lead to a more favorable environment for companies operating in the companion diagnostic market. This regulatory support is likely to facilitate the introduction of new diagnostic tools that align with the latest therapeutic advancements.
Increased Investment in Research and Development
Investment in research and development (R&D) within the pharmaceutical and biotechnology sectors is a critical driver for the companion diagnostic market. Companies are increasingly allocating resources to develop innovative diagnostic solutions that can complement new therapies. In the US, R&D spending in the life sciences sector has seen a steady increase, with estimates suggesting that it could reach $200 billion by 2025. This influx of funding is likely to foster advancements in the companion diagnostic market, leading to the introduction of novel tests that align with emerging therapeutic options.
.png)